Deck 6: The Behavior of Proteins: Enzymes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/78
Play
Full screen (f)
Deck 6: The Behavior of Proteins: Enzymes
1
Which of the following is most directly related to the speed of a reaction?
A) The temperature
B) The DG0 of the reaction
C) The DG of the reaction
D) The DG0‡ of the reaction
E) None of these is correct.
A) The temperature
B) The DG0 of the reaction
C) The DG of the reaction
D) The DG0‡ of the reaction
E) None of these is correct.
D
2
Which of the following is not true?
A) In thermodynamics, spontaneous does not mean instantaneous or even fast.
B) If a reaction is spontaneous then it has a negative DG.
C) Speed of a reaction is a kinetic parameter, not a thermodynamic one.
D) A reaction with a positive DG0 can never happen
A) In thermodynamics, spontaneous does not mean instantaneous or even fast.
B) If a reaction is spontaneous then it has a negative DG.
C) Speed of a reaction is a kinetic parameter, not a thermodynamic one.
D) A reaction with a positive DG0 can never happen
D
3
The kinetic order of a reaction
A) can be determined by inspection from the coefficients of the balanced equation
B) must be determined experimentally
C) always depends on the concentration of enzyme
D) never depends on concentrations of reactants
A) can be determined by inspection from the coefficients of the balanced equation
B) must be determined experimentally
C) always depends on the concentration of enzyme
D) never depends on concentrations of reactants
B
4
First order kinetics means:
A) The rate of a reaction is independent of the amount of reactant measured.
B) The rate of the reaction varies directly with the amount of reactant measured.
C) The rate of the reaction varies with the square of the amount of the reactant measured.
D) More information is needed to answer this question.
E) None of these is correct.
A) The rate of a reaction is independent of the amount of reactant measured.
B) The rate of the reaction varies directly with the amount of reactant measured.
C) The rate of the reaction varies with the square of the amount of the reactant measured.
D) More information is needed to answer this question.
E) None of these is correct.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
5
A transition state analogue
A) binds tightly to the enzyme
B) enhances the activity of the enzyme when bound to it
C) forms a complex with the enzyme that is energetically stable compared to the enzyme-substrate complex
D) will bind to the enzyme by the lock-and-key mechanism rather than the induced-fit mechanism
A) binds tightly to the enzyme
B) enhances the activity of the enzyme when bound to it
C) forms a complex with the enzyme that is energetically stable compared to the enzyme-substrate complex
D) will bind to the enzyme by the lock-and-key mechanism rather than the induced-fit mechanism

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
6
The rate of a reaction is always dependent on the concentration(s) of the reactant(s).

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
7
The sign of Gibb's Free Energy is positive ("+") when energy is released.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
8
The active site of an enzyme
A) is frequently located in a cleft in the enzyme.
B) is the portion of the enzyme to which the substrate binds.
C) contains the reactive groups that catalyze the reaction.
D) all of these
A) is frequently located in a cleft in the enzyme.
B) is the portion of the enzyme to which the substrate binds.
C) contains the reactive groups that catalyze the reaction.
D) all of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
9
Given the rate law,rate = k[A][B],the overall reaction order is
A) zero
B) one
C) two
D) cannot be determined
A) zero
B) one
C) two
D) cannot be determined

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
10
The main difference between a catalyzed and an uncatalyzed reaction is that
A) the activation energy of the catalyzed reaction is lower.
B) the catalyzed reaction has a more favorable free energy change.
C) the catalyzed reaction has a more favorable enthalpy change.
D) the catalyzed reaction has a more favorable entropy change.
A) the activation energy of the catalyzed reaction is lower.
B) the catalyzed reaction has a more favorable free energy change.
C) the catalyzed reaction has a more favorable enthalpy change.
D) the catalyzed reaction has a more favorable entropy change.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
11
Enzymatic activity has an optimum temperature because
A) the component amino acids have varying melting points
B) the rate of reactions is thermodynamically controlled
C) the side chains of essential residues are chemically degraded at higher temperatures
D) raising the temperature speeds up the reaction until protein denaturation sets in
A) the component amino acids have varying melting points
B) the rate of reactions is thermodynamically controlled
C) the side chains of essential residues are chemically degraded at higher temperatures
D) raising the temperature speeds up the reaction until protein denaturation sets in

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
12
The order of a reaction can be determined from the balanced equation for the reaction.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
13
As catalysts,enzymes are
A) significantly less effective than nonenzymatic catalysts
B) slightly less effective than nonenzymatic catalysts
C) significantly more effective than nonenzymatic catalysts
D) slightly more effective than nonenzymatic catalysts
A) significantly less effective than nonenzymatic catalysts
B) slightly less effective than nonenzymatic catalysts
C) significantly more effective than nonenzymatic catalysts
D) slightly more effective than nonenzymatic catalysts

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
14
The rate of a reaction depends on
A) the free energy change
B) the activation energy
C) the enthalpy change
D) the entropy change
A) the free energy change
B) the activation energy
C) the enthalpy change
D) the entropy change

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
15
How much faster is a reaction with the fastest enzyme than without a catalyst?
A) About 10 times faster.
B) About 100 times faster.
C) About 1,000 times faster.
D) About 10,000 times faster.
E) About 1020 times faster.
A) About 10 times faster.
B) About 100 times faster.
C) About 1,000 times faster.
D) About 10,000 times faster.
E) About 1020 times faster.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
16
What effect does a catalyst have on the DG of a reaction?
A) A catalyst lowers the DG.
B) A catalyst raises the DG.
C) A catalyst has no effect on the DG.
D) It depend on the specific catalyst.
A) A catalyst lowers the DG.
B) A catalyst raises the DG.
C) A catalyst has no effect on the DG.
D) It depend on the specific catalyst.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
17
A rate constant is
A) the rate of a reaction at standard temperature and pressure.
B) the rate of a reaction at equilibrium.
C) a proportionality constant relating the rate of a reaction to the concentration(s) of the reactant(s).
D) a kind of transition state.
A) the rate of a reaction at standard temperature and pressure.
B) the rate of a reaction at equilibrium.
C) a proportionality constant relating the rate of a reaction to the concentration(s) of the reactant(s).
D) a kind of transition state.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
18
The amount of energy released during a reaction tells nothing about the rate at which that reaction will occur.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
19
Thermodynamically favorable reactions all release energy.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
20
All catalysts work by lowering the activation energy for a reaction.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
21
The KM expression is equal to
A) (k1 + k2) / k-1
B) (k-1 + k2) / k1
C) (k1 + k-1) / k2
D) k-1 / k1
A) (k1 + k2) / k-1
B) (k-1 + k2) / k1
C) (k1 + k-1) / k2
D) k-1 / k1

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is true about the enzyme chymotrypsin?
A) The enzyme can cleave peptides.
B) The enzyme can cleave esters.
C) The enzyme only binds to aromatic substrates.
D) The enzyme can cleave substrates which are not naturally occurring.
E) All of these are true.
A) The enzyme can cleave peptides.
B) The enzyme can cleave esters.
C) The enzyme only binds to aromatic substrates.
D) The enzyme can cleave substrates which are not naturally occurring.
E) All of these are true.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
23
Most enzyme reactions display first order kinetics for the individual substrates when the substrate concentration is low.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
24
When the substrate concentration is low,an enzyme reaction
A) will display zero-order kinetics.
B) will display first-order kinetics.
C) will display second-order kinetics.
D) will denature and cease to function.
A) will display zero-order kinetics.
B) will display first-order kinetics.
C) will display second-order kinetics.
D) will denature and cease to function.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
25
The substrate will only bind to the enzyme when the shapes fit together rigidly.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
26
When an enzyme is saturated with substrates,
A) it will display zero-order kinetics.
B) it will display first-order kinetics.
C) it will display second-order kinetics.
D) it will denature and cease to function.
A) it will display zero-order kinetics.
B) it will display first-order kinetics.
C) it will display second-order kinetics.
D) it will denature and cease to function.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
27
In the reaction catalyzed by chymotrypsin,a graph in which the rate is plotted against the concentration of substrate
A) is sigmoidal, characteristic of an allosteric enzyme
B) shows that cooperative kinetics are observed
C) shows that the reaction is zero order
D) is hyperbolic, characteristic of a nonallosteric enzyme
A) is sigmoidal, characteristic of an allosteric enzyme
B) shows that cooperative kinetics are observed
C) shows that the reaction is zero order
D) is hyperbolic, characteristic of a nonallosteric enzyme

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
28
The E-S complex often shows as a slight depression in the energy profile for the reaction.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following describes the unique importance of protein Kinase Mæ (PKMæ)?
A) It is a protein kinase
B) It uses ATP to phosphorylate a substrate
C) It is an allosteric enzyme
D) It has been implicated in the formation of long-term memories
A) It is a protein kinase
B) It uses ATP to phosphorylate a substrate
C) It is an allosteric enzyme
D) It has been implicated in the formation of long-term memories

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is implied by induced fit between the enzyme's active site and the substrate?
A) The enzyme is a flexible molecule.
B) An enzyme will work equally well with different substrates.
C) An active site can bind to different substrates.
D) The enzyme is a flexible molecule so different substrates can bind.
E) All of these
A) The enzyme is a flexible molecule.
B) An enzyme will work equally well with different substrates.
C) An active site can bind to different substrates.
D) The enzyme is a flexible molecule so different substrates can bind.
E) All of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
31
The Michaelis-Menten approach to describing the kinetics of an enzyme-catalyzed reaction makes which of the following assumptions about the conversion of product into substrate?
A) The product binds reversibly to the enzyme in order to be converted into the substrate.
B) The product is not converted to substrate to any appreciable extent.
C) The product is converted to substrate following simple first order kinetics.
D) The product is converted to substrate following simple second order kinetics.
A) The product binds reversibly to the enzyme in order to be converted into the substrate.
B) The product is not converted to substrate to any appreciable extent.
C) The product is converted to substrate following simple first order kinetics.
D) The product is converted to substrate following simple second order kinetics.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
32
In the reaction catalyzed by aspartate transcarbamoylase,a graph in which the rate is plotted against the concentration of substrate
A) is sigmoidal, characteristic of an allosteric enzyme
B) shows that noncooperative kinetics are observed
C) shows that the reaction is zero order
D) is hyperbolic, characteristic of a nonallosteric enzyme
A) is sigmoidal, characteristic of an allosteric enzyme
B) shows that noncooperative kinetics are observed
C) shows that the reaction is zero order
D) is hyperbolic, characteristic of a nonallosteric enzyme

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
33
In the induced-fit model of substrate binding to enzymes
A) the substrate changes its conformation to fit the active site
B) the active site changes its conformation to fit the substrate
C) there is a conformational change in the enzyme when the substrate binds
D) there is aggregation of several enzyme molecules when the substrate binds
A) the substrate changes its conformation to fit the active site
B) the active site changes its conformation to fit the substrate
C) there is a conformational change in the enzyme when the substrate binds
D) there is aggregation of several enzyme molecules when the substrate binds

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is true?
A) The E-S complex often dissociates with no reaction taking place.
B) The E-S complex must form before a reaction can take place
C) Once the E-S complex forms, it can go on to form product or dissociate to E + S
D) All of these
A) The E-S complex often dissociates with no reaction taking place.
B) The E-S complex must form before a reaction can take place
C) Once the E-S complex forms, it can go on to form product or dissociate to E + S
D) All of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
35
The active site of an enzyme is the place where the following happens:
A) The enzyme substrate complex forms here.
B) The catalytic reaction happens here.
C) Allosteric regulation of enzyme rate occurs here.
D) The enzyme-substrate complex forms and the reaction occurs at the active site.
E) All of these are correct.
A) The enzyme substrate complex forms here.
B) The catalytic reaction happens here.
C) Allosteric regulation of enzyme rate occurs here.
D) The enzyme-substrate complex forms and the reaction occurs at the active site.
E) All of these are correct.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
36
The reaction catalyzed by aspartate transcarbamoylase is
A) the first step in the synthesis of amino acids.
B) the first step in the synthesis of fatty acids.
C) the first step in the synthesis of CTP and UTP.
D) is part of glycolysis.
A) the first step in the synthesis of amino acids.
B) the first step in the synthesis of fatty acids.
C) the first step in the synthesis of CTP and UTP.
D) is part of glycolysis.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
37
The initial rate of an enzymatic reaction is usually determined in order to assure that
A) the enzyme is active
B) there is no reverse reaction of product to the enzyme-substrate complex
C) the substrate is not used up
D) the experiment can be completed quickly
A) the enzyme is active
B) there is no reverse reaction of product to the enzyme-substrate complex
C) the substrate is not used up
D) the experiment can be completed quickly

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
38
The Michaelis constant is
A) related to the molecular weight of the enzyme
B) a measure of the resistance of the enzyme to denaturation
C) a reflection of the percentage of polar amino acids in the enzyme
D) a measure of how tightly the substrate is bound to the enzyme
A) related to the molecular weight of the enzyme
B) a measure of the resistance of the enzyme to denaturation
C) a reflection of the percentage of polar amino acids in the enzyme
D) a measure of how tightly the substrate is bound to the enzyme

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following are related for a given enzyme?
A) Vmax, KM, and percentage of a-helix
B) Vmax, kcat, and percentage of b-sheet
C) Vmax, kcat, and turnover number
D) Vmax, KM, and molecular weight
A) Vmax, KM, and percentage of a-helix
B) Vmax, kcat, and percentage of b-sheet
C) Vmax, kcat, and turnover number
D) Vmax, KM, and molecular weight

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
40
According to the steady-state assumption
A) the product concentration does not change significantly
B) the substrate concentration is large and does not change significantly
C) the concentration of enzyme-substrate complex remains constant with time
D) the free enzyme concentration is always in great excess to the concentration of enzyme-substrate complex
A) the product concentration does not change significantly
B) the substrate concentration is large and does not change significantly
C) the concentration of enzyme-substrate complex remains constant with time
D) the free enzyme concentration is always in great excess to the concentration of enzyme-substrate complex

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
41
To study the nature of an enzyme,Vmax is not as good a measurement as the catalytic rate constant kcat because:
A) The Vmax is not a true constant since it depends on the concentration of enzyme
B) The Vmax cannot be measured
C) The Vmax is only valid for allosteric enzymes
D) none of these
A) The Vmax is not a true constant since it depends on the concentration of enzyme
B) The Vmax cannot be measured
C) The Vmax is only valid for allosteric enzymes
D) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
42
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
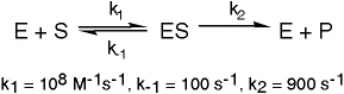 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
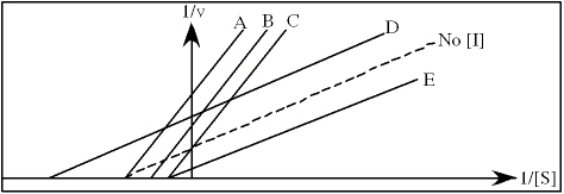
Refer to Exhibit 6A."Restrainin" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.4 nM,the enzyme's apparent KM for the substrate is altered to 100 µM,but the Vmax is unchanged.
In the following graph,which line best represents the Lineweaver-Burk plot obtained in the presence of restrainin?
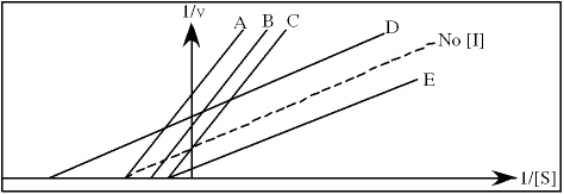
A) A
B) B
C) C
D) D
E) E
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics: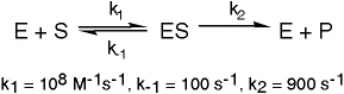 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nMDihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Restrainin" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.4 nM,the enzyme's apparent KM for the substrate is altered to 100 µM,but the Vmax is unchanged.
In the following graph,which line best represents the Lineweaver-Burk plot obtained in the presence of restrainin?

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
43
A Lineweaver-Burk plot is useful in the analysis of enzymatic reactions because
A) it is easier to see whether points deviate from a straight line than from a curve
B) it is not affected by the presence of inhibitors
C) it can be used whether or not the enzyme displays Michaelis-Menten kinetics
D) all of the above
A) it is easier to see whether points deviate from a straight line than from a curve
B) it is not affected by the presence of inhibitors
C) it can be used whether or not the enzyme displays Michaelis-Menten kinetics
D) all of the above

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
44
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
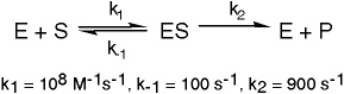 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A.What is the actual velocity of the forward reaction under physiologic conditions?
A) 2 nM/s
B) 45 nM/s
C) 500 nM/s
D) 30 nM/s
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics: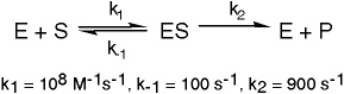 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nMDihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A.What is the actual velocity of the forward reaction under physiologic conditions?
A) 2 nM/s
B) 45 nM/s
C) 500 nM/s
D) 30 nM/s

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
45
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
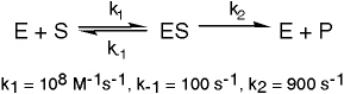 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A.What is the equilibrium constant for the uncatalyzed reaction?
A) 0.9
B) 1.1
C) 2.5
D) Cannot be determined from the information provided.
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics: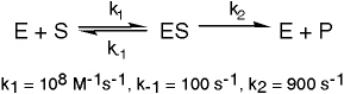 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nMDihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A.What is the equilibrium constant for the uncatalyzed reaction?
A) 0.9
B) 1.1
C) 2.5
D) Cannot be determined from the information provided.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
46
The Michaelis constant determines the Vmax of an enzymatic reaction.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
47
The Michaelis constant is
A) the rate constant for the formation of the substrate-enzyme (E-S) complex.
B) the rate constant for the breakdown of the substrate-enzyme (E-S) complex to form free enzyme and substrate.
C) the rate constant for the breakdown of the substrate-enzyme (E-S) complex to form free enzyme and product.
D) a compilation of several rate constants for the reaction.
A) the rate constant for the formation of the substrate-enzyme (E-S) complex.
B) the rate constant for the breakdown of the substrate-enzyme (E-S) complex to form free enzyme and substrate.
C) the rate constant for the breakdown of the substrate-enzyme (E-S) complex to form free enzyme and product.
D) a compilation of several rate constants for the reaction.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
48
The steady state of an enzyme reaction is the following:
A) The rate observed just after mixing the enzyme and substrate.
B) The rate observed and Vmax.
C) The rate of product formation.
D) The state which exists when E-S complex is forming as fast as it is breaking down.
E) The state which exists when substrate concentration equals KM.
A) The rate observed just after mixing the enzyme and substrate.
B) The rate observed and Vmax.
C) The rate of product formation.
D) The state which exists when E-S complex is forming as fast as it is breaking down.
E) The state which exists when substrate concentration equals KM.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following statements regarding the Michaelis constant is false?
A) It is similar to the affinity constant between the enzyme and substrate.
B) The dimension for the Michaelis constant is concentration, such as molarity.
C) The Michaelis constant determines the Vmax.
D) It is the substrate concentration necessary to reach 1/2 Vmax.
A) It is similar to the affinity constant between the enzyme and substrate.
B) The dimension for the Michaelis constant is concentration, such as molarity.
C) The Michaelis constant determines the Vmax.
D) It is the substrate concentration necessary to reach 1/2 Vmax.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
50
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
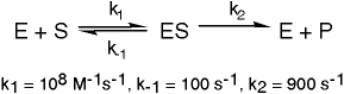 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A.What is the Vmax of the enzyme?
A) 90 nM/s
B) 4500 µM/s
C) 200 µM/s
D) 0.5 M/s
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics: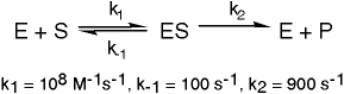 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nMDihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A.What is the Vmax of the enzyme?
A) 90 nM/s
B) 4500 µM/s
C) 200 µM/s
D) 0.5 M/s

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
51
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
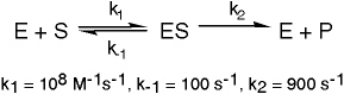 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Hindrate" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.1 nM,the enzyme's KM for the substrate is unchanged,but the apparent Vmax is altered to 50 nM/sec.
In the following graph,which line best represents the Lineweaver-Burk plot obtained in the presence of hindrate?
A) A
B) B
C) C
D) D
E) E
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics: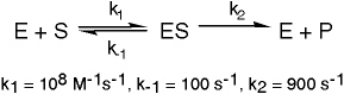 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nMDihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Hindrate" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.1 nM,the enzyme's KM for the substrate is unchanged,but the apparent Vmax is altered to 50 nM/sec.
In the following graph,which line best represents the Lineweaver-Burk plot obtained in the presence of hindrate?

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
52
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
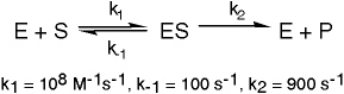 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A.What is the equilibrium constant for the enzyme-catalyzed reaction?
A) 0.9
B) 1.1
C) 2.5
D) Cannot be determined from the information provided.
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics: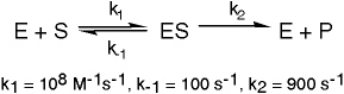 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nMDihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A.What is the equilibrium constant for the enzyme-catalyzed reaction?
A) 0.9
B) 1.1
C) 2.5
D) Cannot be determined from the information provided.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
53
The substrate-enzyme (E-S) complex
A) always proceeds to form the products rapidly.
B) always breaks down to form free enzyme and substrate.
C) always breaks down to form free enzyme and product.
D) may break down to form free enzyme and substrate, or free enzyme and product.
A) always proceeds to form the products rapidly.
B) always breaks down to form free enzyme and substrate.
C) always breaks down to form free enzyme and product.
D) may break down to form free enzyme and substrate, or free enzyme and product.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
54
The drug acetazolamide:
A) Is used to help fight altitude sickness
B) Was found to ruin the taste of carbonated beverages
C) Does not affect the taste of non-carbonated liquors
D) Causes its effect on taste by inhibiting carbonic anhydrase 4
E) All of these
A) Is used to help fight altitude sickness
B) Was found to ruin the taste of carbonated beverages
C) Does not affect the taste of non-carbonated liquors
D) Causes its effect on taste by inhibiting carbonic anhydrase 4
E) All of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
55
If the y-intercept of a Lineweaver-Burk plot = 1.91 (sec/millimole) and the slope = 75.3 L/sec,KM equals:
A) 0.0254 millimolar (mM).
B) 0.523 millimolar (mM).
C) 5.23 millimolar (mM).
D) 39.4 millimolar (mM).
E) 75.3 millimolar (mM).
A) 0.0254 millimolar (mM).
B) 0.523 millimolar (mM).
C) 5.23 millimolar (mM).
D) 39.4 millimolar (mM).
E) 75.3 millimolar (mM).

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
56
It is important that at physiological conditions,enzymes work at Vmax.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
57
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
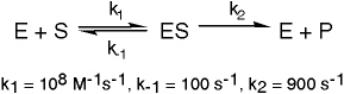 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Hindrate" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.1 nM,the enzyme's KM for the substrate is unchanged,but the apparent Vmax is altered to 50 nM/sec.
A) This is a competitive inhibitor.
B) This is an uncompetitive inhibitor.
C) This is a noncompetitive inhibitor.
D) This is an irreversible inhibitor.
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics: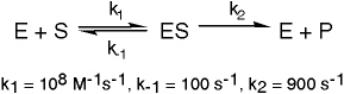 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nMDihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Hindrate" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.1 nM,the enzyme's KM for the substrate is unchanged,but the apparent Vmax is altered to 50 nM/sec.
A) This is a competitive inhibitor.
B) This is an uncompetitive inhibitor.
C) This is a noncompetitive inhibitor.
D) This is an irreversible inhibitor.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
58
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
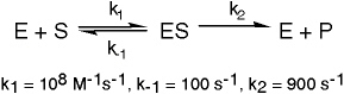 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Restrainin" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.4 nM,the enzyme's apparent KM for the substrate is altered to 100 µM,but the Vmax is unchanged.
A) This is a competitive inhibitor.
B) This is an uncompetitive inhibitor.
C) This is a noncompetitive inhibitor.
D) This is an irreversible inhibitor.
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics: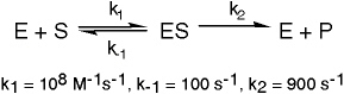 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nMDihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Restrainin" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.4 nM,the enzyme's apparent KM for the substrate is altered to 100 µM,but the Vmax is unchanged.
A) This is a competitive inhibitor.
B) This is an uncompetitive inhibitor.
C) This is a noncompetitive inhibitor.
D) This is an irreversible inhibitor.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
59
If the y-intercept of a Lineweaver-Burk plot = 1.91 (sec/millimole) and the slope = 75.3 L/sec,Vmax equals:
A) 0.0254 millimoles per second.
B) 0.523 millimoles per second.
C) 5.23 millimoles per second.
D) 39.4 millimoles per second.
E) 75.3 millimoles per second.
A) 0.0254 millimoles per second.
B) 0.523 millimoles per second.
C) 5.23 millimoles per second.
D) 39.4 millimoles per second.
E) 75.3 millimoles per second.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
60
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
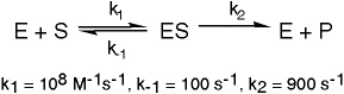 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A.What is the KM of the enzyme?
A) 10 nM
B) 0.1 µM
C) 1 µM
D) 10 µM
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics: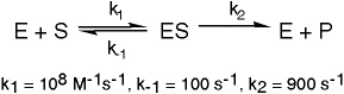 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nMDihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A.What is the KM of the enzyme?
A) 10 nM
B) 0.1 µM
C) 1 µM
D) 10 µM

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
61
Non-competitive inhibitors have this effect:
A) Modifying the KM value.
B) Changing the value for Vmax.
C) Interfering with substrate binding.
D) This type of inhibitor both changes the Vmax and interferes with substrate binding.
E) All of these are correct.
A) Modifying the KM value.
B) Changing the value for Vmax.
C) Interfering with substrate binding.
D) This type of inhibitor both changes the Vmax and interferes with substrate binding.
E) All of these are correct.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
62
What effect is seen on a Lineweaver-Burk graph when a competitive inhibitor is added?
A) The y-intercept is changed, but not change the slope of the line.
B) The slope of the line is changed, but not the y-intercept.
C) Both the y-intercept and the slope of the line are changed.
D) Neither the y-intercept not the slope of the line is changed.
A) The y-intercept is changed, but not change the slope of the line.
B) The slope of the line is changed, but not the y-intercept.
C) Both the y-intercept and the slope of the line are changed.
D) Neither the y-intercept not the slope of the line is changed.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
63
If an inhibitor changes the slope of the Lineweaver-Burk graph,but not the y-intercept,it is this type of inhibition:
A) Competitive.
B) Non-competitive.
C) Mixed Inhibition (uncompetitive inhibition).
D) You cannot tell from the data given.
E) More than one answer is correct.
A) Competitive.
B) Non-competitive.
C) Mixed Inhibition (uncompetitive inhibition).
D) You cannot tell from the data given.
E) More than one answer is correct.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is more likely to inhibit regulatory subunits of an allosteric enzyme?
A) A competitive inhibitor
B) A non-competitive inhibitor
C) An irreversible inhibitor
D) All of these are equally likely to inhibit a regulatory subunit
A) A competitive inhibitor
B) A non-competitive inhibitor
C) An irreversible inhibitor
D) All of these are equally likely to inhibit a regulatory subunit

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
65
If an inhibitor changes the slope of the Lineweaver-Burk graph,but not the x-intercept,it is this type of inhibition:
A) Competitive.
B) Non-competitive.
C) Mixed Inhibition (uncompetitive inhibition).
D) You cannot tell from the data given.
E) More than one answer is correct.
A) Competitive.
B) Non-competitive.
C) Mixed Inhibition (uncompetitive inhibition).
D) You cannot tell from the data given.
E) More than one answer is correct.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
66
Inhibitors can have the following effects on enzyme kinetics:
A) Modifying the KM value.
B) Changing the value for Vmax.
C) Interfering with substrate binding.
D) An inhibitor can change the KM and interfere with substrate binding.
E) All of these are correct.
A) Modifying the KM value.
B) Changing the value for Vmax.
C) Interfering with substrate binding.
D) An inhibitor can change the KM and interfere with substrate binding.
E) All of these are correct.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following diseases has not been successfully treated using the principles of enzyme inhibition?
A) AIDS.
B) Lactose intolerance
C) Virus infection
D) Neither AIDS nor virus infection.
E) All of these have been successfully treated using enzyme inhibitors.
A) AIDS.
B) Lactose intolerance
C) Virus infection
D) Neither AIDS nor virus infection.
E) All of these have been successfully treated using enzyme inhibitors.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following inhibitors binds to the enzyme at a site other than the active site?
A) competitive inhibitor
B) noncompetitive inhibitor
C) irreversible inhibitor
D) all of these
E) none of these
A) competitive inhibitor
B) noncompetitive inhibitor
C) irreversible inhibitor
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
69
Generally speaking,a competitive inhibitor and the substrate cannot both bind to the enzyme at the same time.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
70
The KM of hexokinase for glucose = 0.15 mM and for fructose,KM = 1.5 mM.Which is the preferred substrate?
A) Glucose.
B) Fructose.
C) Neither substrate is preferred over the other.
D) You cannot tell from the data given.
E) None of these answers is correct.
A) Glucose.
B) Fructose.
C) Neither substrate is preferred over the other.
D) You cannot tell from the data given.
E) None of these answers is correct.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
71
What effect is seen on a Lineweaver-Burk graph when a non-competitive inhibitor is added?
A) The y-intercept is changed, but not change the slope of the line.
B) The slope of the line is changed, but not the y-intercept.
C) Both the y-intercept and the slope of the line are changed.
D) Neither the y-intercept not the slope of the line is changed.
A) The y-intercept is changed, but not change the slope of the line.
B) The slope of the line is changed, but not the y-intercept.
C) Both the y-intercept and the slope of the line are changed.
D) Neither the y-intercept not the slope of the line is changed.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
72
Competitive inhibitors have this effect:
A) Modifying the KM value.
B) Changing the value for Vmax.
C) Interfering with substrate binding.
D) This type of inhibitor both changes the KM and interferes with substrate binding.
E) All of these are correct.
A) Modifying the KM value.
B) Changing the value for Vmax.
C) Interfering with substrate binding.
D) This type of inhibitor both changes the KM and interferes with substrate binding.
E) All of these are correct.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
73
Irreversible inhibitors of enzymatic reactions
A) bind to the enzyme only at low temperatures.
B) affect only serine side chains.
C) react with the enzyme to produce a protein that is not enzymatically active and from which the original enzyme cannot be regenerated.
D) are bound to the enzyme by the lock-and-key mechanism.
A) bind to the enzyme only at low temperatures.
B) affect only serine side chains.
C) react with the enzyme to produce a protein that is not enzymatically active and from which the original enzyme cannot be regenerated.
D) are bound to the enzyme by the lock-and-key mechanism.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
74
What effect is seen on a Lineweaver-Burk graph when a mixed-type inhibitor is added?
A) The y-intercept is changed, but not change the slope of the line.
B) The slope of the line is changed, but not the y-intercept.
C) Both the y-intercept and the slope of the line are changed.
D) Neither the y-intercept not the slope of the line is changed.
A) The y-intercept is changed, but not change the slope of the line.
B) The slope of the line is changed, but not the y-intercept.
C) Both the y-intercept and the slope of the line are changed.
D) Neither the y-intercept not the slope of the line is changed.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
75
The value of Vmax changes in
A) competitive inhibition
B) noncompetitive inhibition
C) both forms of inhibition
D) neither form of inhibition
A) competitive inhibition
B) noncompetitive inhibition
C) both forms of inhibition
D) neither form of inhibition

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
76
The fundamental difference between competitive and noncompetitive inhibition is
A) the degree of cooperativity of the reaction
B) the size of the active site of the enzyme
C) the manner of binding of substrate to the enzyme
D) the manner of binding of inhibitor to the enzyme
A) the degree of cooperativity of the reaction
B) the size of the active site of the enzyme
C) the manner of binding of substrate to the enzyme
D) the manner of binding of inhibitor to the enzyme

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
77
A noncompetitive inhibitor
A) binds to the enzyme at a site other than the active site
B) is structurally related to the substrate
C) does not affect the value of Vmax
D) decreases the value of KM
A) binds to the enzyme at a site other than the active site
B) is structurally related to the substrate
C) does not affect the value of Vmax
D) decreases the value of KM

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
78
For competitive inhibition
A) the value of KM decreases
B) the value of Vmax decreases
C) it is possible to overcome the effect of the inhibitor by increasing the concentration of substrate
D) none of the above
A) the value of KM decreases
B) the value of Vmax decreases
C) it is possible to overcome the effect of the inhibitor by increasing the concentration of substrate
D) none of the above

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck


