Exam 6: The Behavior of Proteins: Enzymes
Exam 1: Biochemistry and the Organization of Cells69 Questions
Exam 2: Water: The Solvent for Biochemical Reactions79 Questions
Exam 3: Amino Acids and Peptides77 Questions
Exam 4: The Three-Dimensional Structure of Proteins77 Questions
Exam 5: Protein Purification and Characterization Techniques58 Questions
Exam 6: The Behavior of Proteins: Enzymes78 Questions
Exam 7: The Behavior of Proteins: Enzymes, Mechanisms, and Control77 Questions
Exam 8: Lipids and Proteins Are Associated in Biological Membranes90 Questions
Exam 9: Nucleic Acids: How Structure Conveys Information60 Questions
Exam 10: Biosynthesis of Nucleic Acids: Replication79 Questions
Exam 11: Transcription of the Genetic Code: Biosynthesis of Rna93 Questions
Exam 12: Protein Synthesis: Translation of the Genetic Message80 Questions
Exam 13: Nucleic Acid Biotechnology Techniques89 Questions
Exam 14: Viruses, Cancer, and Immunology35 Questions
Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism56 Questions
Exam 16: Carbohydrates88 Questions
Exam 17: Glycolysis63 Questions
Exam 18: Storage Mechanisms and Control in Carbohydrate Metabolism79 Questions
Exam 19: The Citric Acid Cycle77 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation70 Questions
Exam 21: Lipid Metabolism86 Questions
Exam 22: Photosynthesis74 Questions
Exam 23: The Metabolism of Nitrogen78 Questions
Exam 24: Integration of Metabolism: Cellular Signaling60 Questions
Select questions type
Enzymatic activity has an optimum temperature because
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
D
The rate of a reaction is always dependent on the concentration(s) of the reactant(s).
(True/False)
4.9/5  (37)
(37)
A Lineweaver-Burk plot is useful in the analysis of enzymatic reactions because
(Multiple Choice)
4.7/5  (31)
(31)
Which of the following is more likely to inhibit regulatory subunits of an allosteric enzyme?
(Multiple Choice)
5.0/5  (39)
(39)
Which of the following inhibitors binds to the enzyme at a site other than the active site?
(Multiple Choice)
4.9/5  (46)
(46)
Inhibitors can have the following effects on enzyme kinetics:
(Multiple Choice)
4.8/5  (31)
(31)
If the y-intercept of a Lineweaver-Burk plot = 1.91 (sec/millimole) and the slope = 75.3 L/sec,Vmax equals:
(Multiple Choice)
4.7/5  (30)
(30)
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
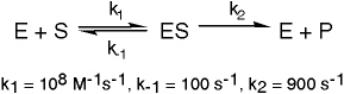 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Hindrate" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.1 nM,the enzyme's KM for the substrate is unchanged,but the apparent Vmax is altered to 50 nM/sec.
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Hindrate" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.1 nM,the enzyme's KM for the substrate is unchanged,but the apparent Vmax is altered to 50 nM/sec.
(Multiple Choice)
4.9/5  (40)
(40)
In the reaction catalyzed by chymotrypsin,a graph in which the rate is plotted against the concentration of substrate
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following statements regarding the Michaelis constant is false?
(Multiple Choice)
4.7/5  (39)
(39)
How much faster is a reaction with the fastest enzyme than without a catalyst?
(Multiple Choice)
4.8/5  (36)
(36)
The amount of energy released during a reaction tells nothing about the rate at which that reaction will occur.
(True/False)
4.9/5  (29)
(29)
The active site of an enzyme is the place where the following happens:
(Multiple Choice)
4.9/5  (34)
(34)
Showing 1 - 20 of 78
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)