Deck 9: Introduction to Solutions and Aqueous Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/178
Play
Full screen (f)
Deck 9: Introduction to Solutions and Aqueous Reactions
1
Give the net ionic equation for the reaction (if any)that occurs when aqueous solutions of H2SO4 and KOH are mixed.
A) H+(aq) + OH-(aq) → H2O(l)
B) 2 K+(aq) + SO42-(aq) → K2SO4(s)
C) H+(aq) + OH-(aq) + 2 K+(aq) + SO42-(aq) → H2O(l) + K2SO4(s)
D) H22+(aq) + OH-(aq) → H2(OH)2(l)
E) No reaction occurs.
A) H+(aq) + OH-(aq) → H2O(l)
B) 2 K+(aq) + SO42-(aq) → K2SO4(s)
C) H+(aq) + OH-(aq) + 2 K+(aq) + SO42-(aq) → H2O(l) + K2SO4(s)
D) H22+(aq) + OH-(aq) → H2(OH)2(l)
E) No reaction occurs.
H+(aq) + OH-(aq) → H2O(l)
2
Give the complete ionic equation for the reaction (if any)that occurs when aqueous solutions of MgSO3 and HI are mixed.
A) 2 H+(aq) + SO32-(aq) → H2SO3(s)
B) Mg2+(aq) + 2 I-(aq) → MgI2(s)
C) 2 H+(aq) + SO32-(aq) + Mg2+(aq) + 2 I-(aq)→ H2SO3(s) + MgI2(aq)
D) 2 H+(aq) + SO32-(aq) → H2O(l) + SO2(g)
E) No reaction occurs.
A) 2 H+(aq) + SO32-(aq) → H2SO3(s)
B) Mg2+(aq) + 2 I-(aq) → MgI2(s)
C) 2 H+(aq) + SO32-(aq) + Mg2+(aq) + 2 I-(aq)→ H2SO3(s) + MgI2(aq)
D) 2 H+(aq) + SO32-(aq) → H2O(l) + SO2(g)
E) No reaction occurs.
2 H+(aq) + SO32-(aq) → H2O(l) + SO2(g)
3
Determine the molarity of a solution formed by dissolving 97.7 g LiBr in enough water to yield 750.0 mL of solution.
A) 1.50 M
B) 1.18 M
C) 0.130 M
D) 0.768 M
E) 2.30 M
A) 1.50 M
B) 1.18 M
C) 0.130 M
D) 0.768 M
E) 2.30 M
1.50 M
4
How many molecules of sucrose (C12H22O11,molar mass = 342.30 g/mol)are contained in 14.3 mL of 0.140 M sucrose solution?
A) 8.29 × 1022 molecules C12H22O11
B) 1.21 × 1021 molecules C12H22O11
C) 6.15 × 1022 molecules C12H22O11
D) 1.63 × 1023 molecules C12H22O11
E) 5.90 × 1024 molecules C12H22O11
A) 8.29 × 1022 molecules C12H22O11
B) 1.21 × 1021 molecules C12H22O11
C) 6.15 × 1022 molecules C12H22O11
D) 1.63 × 1023 molecules C12H22O11
E) 5.90 × 1024 molecules C12H22O11

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
5
Give the net ionic equation for the reaction (if any)that occurs when aqueous solutions of Al(C2H3O2)3 and LiNO3 are mixed.
A) Al3+(aq) + 3 NO3-(aq) → Al(NO3)3(s)
B) Li+(aq) + C2H3O2-(aq) → LiC2H3O2(s)
C) Al3+(aq) + 3 NO3-(aq) + Li+(aq) + C2H3O2-(aq) → Al(NO3)3(aq) + LiC2H3O2(s)
D) 3 Li+(aq) + (C2H3O2)33-(aq) → Li3(C2H3O2)3(s)
E) No reaction occurs.
A) Al3+(aq) + 3 NO3-(aq) → Al(NO3)3(s)
B) Li+(aq) + C2H3O2-(aq) → LiC2H3O2(s)
C) Al3+(aq) + 3 NO3-(aq) + Li+(aq) + C2H3O2-(aq) → Al(NO3)3(aq) + LiC2H3O2(s)
D) 3 Li+(aq) + (C2H3O2)33-(aq) → Li3(C2H3O2)3(s)
E) No reaction occurs.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
6
According to the following reaction,what mass of PbCl2 can form from 235 mL of 0.110 M KCl solution? Assume that there is excess Pb(NO3)2.
2 KCl(aq)+ Pb(NO3)2(aq)→ PbCl2(s)+ 2 KNO3(aq)
A) 7.19 g
B) 3.59 g
C) 1.80 g
D) 5.94 g
E) 1.30 g
2 KCl(aq)+ Pb(NO3)2(aq)→ PbCl2(s)+ 2 KNO3(aq)
A) 7.19 g
B) 3.59 g
C) 1.80 g
D) 5.94 g
E) 1.30 g

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
7
Identify acetic acid.
A) strong electrolyte, weak acid
B) weak electrolyte, weak acid
C) strong electrolyte, strong acid
D) weak electrolyte, strong acid
E) nonelectrolyte
A) strong electrolyte, weak acid
B) weak electrolyte, weak acid
C) strong electrolyte, strong acid
D) weak electrolyte, strong acid
E) nonelectrolyte

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
8
What volume of 0.305 M AgNO3 is required to react exactly with 155.0 mL of 0.274 M Na2SO4 solution? Hint: You will want to write a balanced reaction.
A) 581 mL
B) 173 mL
C) 345 mL
D) 139 mL
E) 278 mL
A) 581 mL
B) 173 mL
C) 345 mL
D) 139 mL
E) 278 mL

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
9
Give the net ionic equation for the reaction (if any)that occurs when aqueous solutions of Na2CO3 and HCl are mixed.
A) 2 H+(aq) + CO32-(aq) → H2CO3(s)
B) 2 Na+(aq) + CO32-(aq) + 2 H+(aq) + 2 Cl-(aq) → H2CO3(s) + 2 NaCl(aq)
C) 2 H+(aq) + CO32-(aq) → H2O(l) + CO2(g)
D) 2 Na+(aq) + CO32-(aq) + 2 H+(aq) + 2 Cl-(aq) → H2CO3(s) + 2 Na+(aq) + 2 Cl-(aq)
E) No reaction occurs.
A) 2 H+(aq) + CO32-(aq) → H2CO3(s)
B) 2 Na+(aq) + CO32-(aq) + 2 H+(aq) + 2 Cl-(aq) → H2CO3(s) + 2 NaCl(aq)
C) 2 H+(aq) + CO32-(aq) → H2O(l) + CO2(g)
D) 2 Na+(aq) + CO32-(aq) + 2 H+(aq) + 2 Cl-(aq) → H2CO3(s) + 2 Na+(aq) + 2 Cl-(aq)
E) No reaction occurs.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
10
Give the net ionic equation for the reaction (if any)that occurs when aqueous solutions of K2S and Fe(NO3)2 are mixed.
A) K+(aq) + NO3-(aq) → KNO3(s)
B) Fe2+(aq) + S2-(aq) + 2 K+(aq) + 2 NO3-(aq) → FeS(s) + 2 K+(aq) + 2 NO3-(aq)
C) Fe2+(aq) + S2-(aq) + 2 K+(aq) + 2 NO3-(aq) → Fe2+(aq) + S2-(aq) + 2 KNO3(s)
D) Fe2+(aq) + S2-(aq) → FeS(s)
E) No reaction occurs.
A) K+(aq) + NO3-(aq) → KNO3(s)
B) Fe2+(aq) + S2-(aq) + 2 K+(aq) + 2 NO3-(aq) → FeS(s) + 2 K+(aq) + 2 NO3-(aq)
C) Fe2+(aq) + S2-(aq) + 2 K+(aq) + 2 NO3-(aq) → Fe2+(aq) + S2-(aq) + 2 KNO3(s)
D) Fe2+(aq) + S2-(aq) → FeS(s)
E) No reaction occurs.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
11
Give the complete ionic equation for the reaction (if any)that occurs when aqueous solutions of lithium sulfide and copper (II)nitrate are mixed.
A) Li+(aq) + SO42-(aq) + Cu+(aq) + NO3-(aq) → CuS(s) + Li+(aq) + NO3-(aq)
B) Li+(aq) + S-(aq) + Cu+(aq) + NO3-(aq) → CuS(s) + LiNO3(aq)
C) 2 Li+(aq) + S2-(aq) + Cu2+(aq) + 2 NO3-(aq) → Cu2+(aq) + S2-(aq) + 2 LiNO3(s)
D) 2 Li+(aq) + S2-(aq) + Cu2+(aq) + 2 NO3-(aq) → CuS(s) + 2 Li+(aq) + 2 NO3-(aq)
E) No reaction occurs.
A) Li+(aq) + SO42-(aq) + Cu+(aq) + NO3-(aq) → CuS(s) + Li+(aq) + NO3-(aq)
B) Li+(aq) + S-(aq) + Cu+(aq) + NO3-(aq) → CuS(s) + LiNO3(aq)
C) 2 Li+(aq) + S2-(aq) + Cu2+(aq) + 2 NO3-(aq) → Cu2+(aq) + S2-(aq) + 2 LiNO3(s)
D) 2 Li+(aq) + S2-(aq) + Cu2+(aq) + 2 NO3-(aq) → CuS(s) + 2 Li+(aq) + 2 NO3-(aq)
E) No reaction occurs.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
12
Determine the molarity of a solution formed by dissolving 468 mg of MgI2 in enough water to yield 50.0 mL of solution.
A) 0.0297 M
B) 0.0337 M
C) 0.0936 M
D) 0.0107 M
E) 0.0651 M
A) 0.0297 M
B) 0.0337 M
C) 0.0936 M
D) 0.0107 M
E) 0.0651 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
13
Identify HCl.
A) strong electrolyte, weak acid
B) weak electrolyte, weak acid
C) strong electrolyte, strong acid
D) weak electrolyte, strong acid
E) nonelectrolyte
A) strong electrolyte, weak acid
B) weak electrolyte, weak acid
C) strong electrolyte, strong acid
D) weak electrolyte, strong acid
E) nonelectrolyte

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is an acid-base reaction?
A) C(s) + O2(g) → CO2(g)
B) 2 HClO4(aq) + Ca(OH)2(aq) → 2 H2O(l) + Ca(ClO4)2(aq)
C) Fe(s) + 2 AgNO3(aq) → 2 Ag(s) + Fe(NO3)2(aq)
D) MgSO4(aq) + Ba(NO3)2(aq) → Mg(NO3)2(aq) + BaSO4(s)
E) None of the above are acid base reactions.
A) C(s) + O2(g) → CO2(g)
B) 2 HClO4(aq) + Ca(OH)2(aq) → 2 H2O(l) + Ca(ClO4)2(aq)
C) Fe(s) + 2 AgNO3(aq) → 2 Ag(s) + Fe(NO3)2(aq)
D) MgSO4(aq) + Ba(NO3)2(aq) → Mg(NO3)2(aq) + BaSO4(s)
E) None of the above are acid base reactions.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
15
Determine the number of grams H2 formed when 250.0 mL of 0.743 M HCl solution reacts with 3.41 × 1023 atoms of Fe according to the following reaction. 2 HCl(aq)+ Fe(s)→ H2(g)+ FeCl2(aq)
A) 0.374 g
B) 1.33 g
C) 1.14 g
D) 0.187 g
E) 1.51 g
A) 0.374 g
B) 1.33 g
C) 1.14 g
D) 0.187 g
E) 1.51 g

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a gas-evolution reaction?
A) 2 C2H6(l) + 7 O2(g) → 4 CO2(g) + 6 H2O(g)
B) 2 H2(g) + O2(g) → 2 H2O(g)
C) LiCl(aq) + NaNO3(aq) → LiNO3(aq) + NaCl(g)
D) NH4Cl(aq) + KOH(aq) → KCl(aq) + NH3(g) + H2O(l)
E) None of the above are gas-evolution reactions.
A) 2 C2H6(l) + 7 O2(g) → 4 CO2(g) + 6 H2O(g)
B) 2 H2(g) + O2(g) → 2 H2O(g)
C) LiCl(aq) + NaNO3(aq) → LiNO3(aq) + NaCl(g)
D) NH4Cl(aq) + KOH(aq) → KCl(aq) + NH3(g) + H2O(l)
E) None of the above are gas-evolution reactions.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
17
What mass (in g)of AgCl is formed from the reaction of 75.0 mL of a 0.078 M AgC2H3O2 solution with 55.0 mL of 0.109 M MgCl2 solution?
2 AgC2H3O2(aq)+ MgCl2(aq)→ 2 AgCl(s)+ Mg(C2H3O2)2(aq)
A) 0.838 g
B) 1.72 g
C) 0.859 g
D) 2.56 g
E) 1.70 g
2 AgC2H3O2(aq)+ MgCl2(aq)→ 2 AgCl(s)+ Mg(C2H3O2)2(aq)
A) 0.838 g
B) 1.72 g
C) 0.859 g
D) 2.56 g
E) 1.70 g

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
18
According to the following reaction,how many moles of Fe(OH)2 can form from 175.0 mL of 0.227 M LiOH solution? Assume that there is excess FeCl2.
FeCl2(aq)+ 2 LiOH(aq)→ Fe(OH)2(s)+ 2 LiCl(aq)
A) 3.97 × 10-2 moles
B) 2.52 × 10-2 moles
C) 1.99 × 10-2 moles
D) 5.03 × 10-2 moles
E) 6.49 × 10-2 moles
FeCl2(aq)+ 2 LiOH(aq)→ Fe(OH)2(s)+ 2 LiCl(aq)
A) 3.97 × 10-2 moles
B) 2.52 × 10-2 moles
C) 1.99 × 10-2 moles
D) 5.03 × 10-2 moles
E) 6.49 × 10-2 moles

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
19
Choose the statement below that is TRUE.
A) A weak acid solution consists of mostly nonionized acid molecules.
B) The term "strong electrolyte" means that the substance is extremely reactive.
C) A strong acid solution consists of only partially ionized acid molecules.
D) The term "weak electrolyte" means that the substance is inert.
E) A molecular compound that does not ionize in solution is considered a strong electrolyte.
A) A weak acid solution consists of mostly nonionized acid molecules.
B) The term "strong electrolyte" means that the substance is extremely reactive.
C) A strong acid solution consists of only partially ionized acid molecules.
D) The term "weak electrolyte" means that the substance is inert.
E) A molecular compound that does not ionize in solution is considered a strong electrolyte.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
20
According to the following reaction,what volume of 0.244 M KCl solution is required to react exactly with 50.0 mL of 0.210 M Pb(NO3)2 solution?
2 KCl(aq)+ Pb(NO3)2(aq)→ PbCl2(s)+ 2 KNO3(aq)
A) 97.4 mL
B) 116 mL
C) 43.0 mL
D) 86.1 mL
E) 58.1 mL
2 KCl(aq)+ Pb(NO3)2(aq)→ PbCl2(s)+ 2 KNO3(aq)
A) 97.4 mL
B) 116 mL
C) 43.0 mL
D) 86.1 mL
E) 58.1 mL

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following compounds will undergo a gas-evolving reaction in an aqueous solution?
A) CH3COOH
B) HF
C) HCl
D) H2SO4
E) H2CO3
A) CH3COOH
B) HF
C) HCl
D) H2SO4
E) H2CO3

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
22
A beaker contains 0.50 mol of potassium bromide in 600 mL of water.An additional 600 mL of water is added.The number of moles of potassium bromide in the beaker is
A) 0.50 mol.
B) 0.83 mol.
C) 0.25 mol.
D) 1.2 mol.
E) 0.42 mol.
A) 0.50 mol.
B) 0.83 mol.
C) 0.25 mol.
D) 1.2 mol.
E) 0.42 mol.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is a strong electrolyte in solution?
A) CdS
B) FeCl3
C) CH3COOH
D) NiC2O4
E) PbMnO4
A) CdS
B) FeCl3
C) CH3COOH
D) NiC2O4
E) PbMnO4

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
24
If 4.89 g of ZnCl2 is dissolved in enough water to give a total volume of 500 mL,what is the molarity of the solution?
A) 0.217 M
B) 0.0179 M
C) 0.0717 M
D) 1.33 M
E) 0.849 M
A) 0.217 M
B) 0.0179 M
C) 0.0717 M
D) 1.33 M
E) 0.849 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
25
6.74 g of the monoprotic acid KHP (MW = 204.2 g/mol)is dissolved into water.The sample is titrated with a 0.703 M solution of calcium hydroxide to the equivalence point.What volume of base was used?
A) 8.64 mL
B) 11.8 mL
C) 23.5 mL
D) 47.0 mL
E) 93.9 mL
A) 8.64 mL
B) 11.8 mL
C) 23.5 mL
D) 47.0 mL
E) 93.9 mL

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
26
Determine the oxidation state of P in PO33-.
A) +3
B) +6
C) +2
D) 0
E) -3
A) +3
B) +6
C) +2
D) 0
E) -3

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
27
What element is undergoing reduction (if any)in the following reaction?
Zn(s)+ 2 AgNO3(aq)→ Zn(NO3)2(aq)+ 2 Ag(s)
A) Zn
B) N
C) O
D) Ag
E) This is not an oxidation-reduction reaction.
Zn(s)+ 2 AgNO3(aq)→ Zn(NO3)2(aq)+ 2 Ag(s)
A) Zn
B) N
C) O
D) Ag
E) This is not an oxidation-reduction reaction.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
28
Determine the reducing agent in the following reaction. 2 Li(s)+ Fe(C2H3O2)2(aq)→ 2 LiC2H3O2(aq)+ Fe(s)
A) O
B) H
C) C
D) Fe
E) Li
A) O
B) H
C) C
D) Fe
E) Li

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
29
Determine the oxidation state of nitrogen in NO.
A) +5
B) +3
C) 0
D) +2
E) +4
A) +5
B) +3
C) 0
D) +2
E) +4

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
30
The titration of 80.0 mL of an unknown concentration H3PO4 solution requires 126 mL of 0.218 M KOH solution.What is the concentration of the H3PO4 solution (in M)?
A) 1.03 M
B) 0.343 M
C) 0.114 M
D) 0.138 M
E) 0.0461 M
A) 1.03 M
B) 0.343 M
C) 0.114 M
D) 0.138 M
E) 0.0461 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
31
Determine the oxidation state of nitrogen in NO2.
A) +5
B) +3
C) 0
D) +2
E) +4
A) +5
B) +3
C) 0
D) +2
E) +4

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
32
What element is undergoing oxidation (if any)in the following reaction?
CH4(g)+ 2 O2(g)→ CO2(g)+ 2 H2O(g)
A) O
B) H
C) C
D) both C and H
E) None of the elements is undergoing oxidation.
CH4(g)+ 2 O2(g)→ CO2(g)+ 2 H2O(g)
A) O
B) H
C) C
D) both C and H
E) None of the elements is undergoing oxidation.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
33
Determine the oxidizing agent in the following reaction. Ni(s)+ 2 AgClO4(aq)→ Ni(ClO4)2(aq)+ 2 Ag(s)
A) Ag
B) Ni
C) Cl
D) O
E) This is not an oxidation-reduction reaction.
A) Ag
B) Ni
C) Cl
D) O
E) This is not an oxidation-reduction reaction.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
34
When an aqueous solution of manganese (II)nitrate is combined with an aqueous solution of ammonium sulfide,what should precipitate out?
A) MnS
B) Mn(SO3)2
C) Mn(SO4)2
D) Mn2SO3
E) Mn2SO4
A) MnS
B) Mn(SO3)2
C) Mn(SO4)2
D) Mn2SO3
E) Mn2SO4

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
35
What element is undergoing oxidation (if any)in the following reaction?
2 MnO4-(aq)+ 3 NO2-(aq)+ H2O(l)→ 2 MnO2(s)+ 3 NO3-(aq)+ 2 OH-(aq)
A) Mn
B) O
C) N
D) H
E) This is not an oxidation-reduction reaction
2 MnO4-(aq)+ 3 NO2-(aq)+ H2O(l)→ 2 MnO2(s)+ 3 NO3-(aq)+ 2 OH-(aq)
A) Mn
B) O
C) N
D) H
E) This is not an oxidation-reduction reaction

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
36
What is the name for an aqueous solution of HIO?
A) hydroiodic acid
B) hypoiodous acid
C) iodous acid
D) iodic acid
E) periodic acid
A) hydroiodic acid
B) hypoiodous acid
C) iodous acid
D) iodic acid
E) periodic acid

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
37
Determine the oxidation state of C in CO3-2.
A) +4
B) +2
C) -2
D) -4
E) +6
A) +4
B) +2
C) -2
D) -4
E) +6

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
38
The titration of 25.0 mL of an unknown concentration H2SO4 solution requires 83.6 mL of 0.12 M LiOH solution.What is the concentration of the H2SO4 solution (in M)?
A) 0.20 M
B) 0.40 M
C) 0.10 M
D) 0.36 M
E) 0.25 M
A) 0.20 M
B) 0.40 M
C) 0.10 M
D) 0.36 M
E) 0.25 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
39
What element is undergoing oxidation (if any)in the following reaction?
Zn(s)+ 2 AgNO3(aq)→ Zn(NO3)2(aq)+ 2 Ag(s)
A) Zn
B) N
C) O
D) Ag
E) This is not an oxidation-reduction reaction.
Zn(s)+ 2 AgNO3(aq)→ Zn(NO3)2(aq)+ 2 Ag(s)
A) Zn
B) N
C) O
D) Ag
E) This is not an oxidation-reduction reaction.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
40
All of the following compounds are soluble EXCEPT
A) Ba(OH)2.
B) Pb(NO3)2.
C) ZnCl2.
D) AgBr.
E) Cu(C2H3O2)2.
A) Ba(OH)2.
B) Pb(NO3)2.
C) ZnCl2.
D) AgBr.
E) Cu(C2H3O2)2.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
41
What element is undergoing oxidation (if any)in the following reaction?
2 HCl(aq)+ Ca(OH)2(aq)→ 2 H2O(l)+ 2 CaCl2(aq)
A) H
B) Cl
C) Ca
D) O
E) This is not an oxidation-reduction reaction
2 HCl(aq)+ Ca(OH)2(aq)→ 2 H2O(l)+ 2 CaCl2(aq)
A) H
B) Cl
C) Ca
D) O
E) This is not an oxidation-reduction reaction

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
42
How many liters of a 0.0550 M Li F solution contain 0.163 moles of Li F?
A) 3.37 L
B) 1.48 L
C) 8.97 L
D) 2.96 L
E) 1.12 L
A) 3.37 L
B) 1.48 L
C) 8.97 L
D) 2.96 L
E) 1.12 L

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
43
How many moles of Na I are required to make 250 mL of a 3.00 M solution?
A) 750 moles
B) 0.750 moles
C) 3 moles
D) 0.250 moles
A) 750 moles
B) 0.750 moles
C) 3 moles
D) 0.250 moles

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
44
How many grams of AgNO3 are needed to make 250.mL of a solution that is 0.155 M?
A) 0.105 g
B) 0.152 g
C) 6.58 g
D) 105 g
A) 0.105 g
B) 0.152 g
C) 6.58 g
D) 105 g

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
45
Determine the oxidation state of Cl in ClO3-.
A) +1
B) +3
C) +5
D) +7
E) 0
A) +1
B) +3
C) +5
D) +7
E) 0

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
46
What volume of 1.46 M CrBr2 solution is required to react with 50.0 mL of 0.210 M Na2CO3 solution?
CrBr2(aq)+ Na2CO3(aq)→ CrCO3(s)+ 2NaBr(aq)
A) 139 mL
B) 45.3 mL
C) 7.19 mL
D) 1.68 mL
E) 34.6 mL
CrBr2(aq)+ Na2CO3(aq)→ CrCO3(s)+ 2NaBr(aq)
A) 139 mL
B) 45.3 mL
C) 7.19 mL
D) 1.68 mL
E) 34.6 mL

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
47
Calculate the number of grams of solute in 250.0 mL of 0.179 M KOH.
A) 78.4
B) 0.798
C) 2.51 ×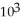
D) 2.51
E) 7.98 × 10-4
A) 78.4
B) 0.798
C) 2.51 ×
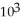
D) 2.51
E) 7.98 × 10-4

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
48
How many milliliters of a 0.266 M CsNO3 solution are required to make 150.0 mL of 0.075 M CsNO3 solution?
A) 53.2 mL
B) 42.3 mL
C) 18.8 mL
D) 23.6 mL
E) 35.1 mL
A) 53.2 mL
B) 42.3 mL
C) 18.8 mL
D) 23.6 mL
E) 35.1 mL

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
49
How many moles of Na F are contained in 258.6 mL of 0.0296 M Na F solution?
A) 1.31 × 10-3 mol
B) 8.74 × 10-3 mol
C) 1.14 × 10-3 mol
D) 3.67 × 10-3 mol
E) 7.65 × 10-3 mol
A) 1.31 × 10-3 mol
B) 8.74 × 10-3 mol
C) 1.14 × 10-3 mol
D) 3.67 × 10-3 mol
E) 7.65 × 10-3 mol

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
50
If 42.389 g of Fe3Br2 is dissolved in enough water to give a total volume of 750 mL,what is the molarity of the solution?
A) 0.327 M
B) 0.0971 M
C) 0.0327 M
D) 0.173M
E) 0.436 M
A) 0.327 M
B) 0.0971 M
C) 0.0327 M
D) 0.173M
E) 0.436 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
51
How many grams of NaCl are required to make 300.0 mL of a 2.500 M solution?
A) 58.40 g
B) 175.3 g
C) 14.60 g
D) 43.83 g
A) 58.40 g
B) 175.3 g
C) 14.60 g
D) 43.83 g

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
52
Determine the concentration of a solution prepared by diluting 25.0 mL of a stock 0.188 M Sr(NO3)2 solution to 150.0 mL.
A) 1.13 M
B) 0.0887 M
C) 0.0313 M
D) 0.0199 M
E) 0.0501 M
A) 1.13 M
B) 0.0887 M
C) 0.0313 M
D) 0.0199 M
E) 0.0501 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
53
How many milliliters of a 0.184 M RbNO3 solution contain 0.113 moles of RbNO3?
A) 543 mL
B) 163 mL
C) 614 mL
D) 885 mL
E) 326 mL
A) 543 mL
B) 163 mL
C) 614 mL
D) 885 mL
E) 326 mL

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following solutions will have the highest concentration of chloride ions?
A) 0.10 M NaCl
B) 0.10 M MgCl2
C) 0.10 M AlCl3
D) 0.05 M CaCl2
E) All of these solutions have the same concentration of chloride ions.
A) 0.10 M NaCl
B) 0.10 M MgCl2
C) 0.10 M AlCl3
D) 0.05 M CaCl2
E) All of these solutions have the same concentration of chloride ions.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
55
How many moles of CH3CH2Cl are contained in 548 mL of 0.0788 M CH3CH2Cl solution?
A) 4.32 × 10-2 mol
B) 2.32 × 10-2 mol
C) 6.95 × 10-2 mol
D) 1.44 × 10-2 mol
E) 5.26 × 10-2 mol
A) 4.32 × 10-2 mol
B) 2.32 × 10-2 mol
C) 6.95 × 10-2 mol
D) 1.44 × 10-2 mol
E) 5.26 × 10-2 mol

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
56
What is the concentration of nitrate ions in a 0.125 M Ba(NO3)2 solution?
A) 0.125 M
B) 0.0625 M
C) 0.375 M
D) 0.250 M
E) 0.160 M
A) 0.125 M
B) 0.0625 M
C) 0.375 M
D) 0.250 M
E) 0.160 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
57
What volume (in mL)of 0.0887 M Mg Br2 solution is needed to make 275.0 mL of 0.0224 M Mg Br2 solution?
A) 72.3 mL
B) 91.8 mL
C) 10.9 mL
D) 69.4 mL
E) 14.4 mL
A) 72.3 mL
B) 91.8 mL
C) 10.9 mL
D) 69.4 mL
E) 14.4 mL

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
58
Determine the concentration of a solution prepared by diluting 20.0 mL of a 0.200 M KCl to 250.0 mL.
A) 0.160 M
B) 0.0320 M
C) 2.50 M
D) 0.00800 M
E) 0.0160 M
A) 0.160 M
B) 0.0320 M
C) 2.50 M
D) 0.00800 M
E) 0.0160 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
59
What is the concentration of magnesium ions in a 0.125 M Mg SO4 solution?
A) 0.125 M
B) 0.0625 M
C) 0.375 M
D) 0.250 M
E) 0.160 M
A) 0.125 M
B) 0.0625 M
C) 0.375 M
D) 0.250 M
E) 0.160 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
60
What is the concentration of FeCl3 in a solution prepared by dissolving 30.0 g of FeCl3 in enough water to make 275 mL of solution?
A) 6.73 × 10-4 M
B) 0. 673 M
C) 1.49 M
D) 1.49 × 103 M
A) 6.73 × 10-4 M
B) 0. 673 M
C) 1.49 M
D) 1.49 × 103 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
61
What volume of a 0.540 M NaOH solution contains 12.5 g of NaOH?
A) 0. 169 L
B) 0. 579 L
C) 1. 73 L
D) 5.92 L
A) 0. 169 L
B) 0. 579 L
C) 1. 73 L
D) 5.92 L

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
62
How many grams of C 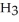 OH must be added to water to prepare 175mL of a solution that is 1.0 M CH3OH?
OH must be added to water to prepare 175mL of a solution that is 1.0 M CH3OH?
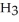 OH must be added to water to prepare 175mL of a solution that is 1.0 M CH3OH?
OH must be added to water to prepare 175mL of a solution that is 1.0 M CH3OH?
Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
63
What is the concentration of NO3- ions in a solution prepared by dissolving 15.0 g of Ca(NO3)2 in enough water to produce 300.mL of solution?
A) 0. 152 M
B) 0. 305 M
C) 0. 403 M
D) 0.609 M
A) 0. 152 M
B) 0. 305 M
C) 0. 403 M
D) 0.609 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
64
What is the concentration of HCl in the final solution when 65 mL of a 9.0 M HCl solution is diluted with pure water to a total volume of 0.15 L?
A) 2.1 × 10-2 M
B) 3.9 M
C) 21 M
D) 3.9 × 103 M
A) 2.1 × 10-2 M
B) 3.9 M
C) 21 M
D) 3.9 × 103 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
65
How many grams of NaOH (MW = 40.0)are there in 500.0 mL of a 0.175 M NaOH solution?

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
66
How many aluminum ions are present in 65.5 mL of 0.210 M Al I3 solution?
A) 4.02 × 1023 aluminum ions
B) 5.79 × 1024 aluminum ions
C) 2.48 × 1022 aluminum ions
D) 8.28 × 1021 aluminum ions
E) 1.21 × 1022 aluminum ions
A) 4.02 × 1023 aluminum ions
B) 5.79 × 1024 aluminum ions
C) 2.48 × 1022 aluminum ions
D) 8.28 × 1021 aluminum ions
E) 1.21 × 1022 aluminum ions

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
67
How many ions are present in 30.0 mL of 0.600 M H2CO3 solution?
A) 3.25 × 1022 ions
B) 2.17 × 1022 ions
C) 1.08 × 1022 ions
D) 5.42 × 1022 ions
E) 3.61 × 1021 ions
A) 3.25 × 1022 ions
B) 2.17 × 1022 ions
C) 1.08 × 1022 ions
D) 5.42 × 1022 ions
E) 3.61 × 1021 ions

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
68
If the reaction of phosphate ion with water is ignored,what is the total concentration of ions in a solution prepared by dissolving 7.00 g of K3PO4 in enough water to make 350.mL of solution?
A) 0.0 236 M
B) 0. 0943 M
C) 0. 377 M
D) 0. 754 M
A) 0.0 236 M
B) 0. 0943 M
C) 0. 377 M
D) 0. 754 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
69
How many milliliters of a 9.0 M H2SO4 solution are needed to make 0.45 L of a 3.5 M solution?
A) 0. 18 mL
B) 1.2 mL
C) 180 mL
D) 1200 mL
A) 0. 18 mL
B) 1.2 mL
C) 180 mL
D) 1200 mL

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
70
What is the concentration (M)of C 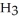 OH in a solution prepared by dissolving 3.96 g of C
OH in a solution prepared by dissolving 3.96 g of C 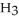 OH in sufficient water to give exactly 230 mL of solution?
OH in sufficient water to give exactly 230 mL of solution?
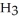 OH in a solution prepared by dissolving 3.96 g of C
OH in a solution prepared by dissolving 3.96 g of C 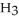 OH in sufficient water to give exactly 230 mL of solution?
OH in sufficient water to give exactly 230 mL of solution?
Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
71
What is the concentration (M)of a NaCl solution prepared by dissolving 5.3 g of NaCl in sufficient water to give 115 mL of solution?

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
72
How many chloride ions are present in 65.5 mL of 0.210 M Al Cl3 solution?
A) 4.02 × 1023 chloride ions
B) 5.79 × 1024 chloride ions
C) 2.48 × 1022 chloride ions
D) 8.28 × 1021 chloride ions
E) 1.21 × 1022 chloride ions
A) 4.02 × 1023 chloride ions
B) 5.79 × 1024 chloride ions
C) 2.48 × 1022 chloride ions
D) 8.28 × 1021 chloride ions
E) 1.21 × 1022 chloride ions

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
73
How many milliliters of a stock solution of 12.1 M 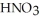 would be needed to prepare 0.500 L of 0.500 M
would be needed to prepare 0.500 L of 0.500 M 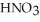 ?
?
A) 0.0484
B) 20.7
C) 3.03
D) 48.4
E) 0.0207
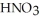 would be needed to prepare 0.500 L of 0.500 M
would be needed to prepare 0.500 L of 0.500 M 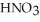 ?
?A) 0.0484
B) 20.7
C) 3.03
D) 48.4
E) 0.0207

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
74
A FeCl3 solution is 0.175 M.How many mL of a 0.175 M FeCl3 solution are needed to make 650.mL of a solution that is 0.300 M in Cl- ion?
A) 1.11 mL
B) 371 mL
C) 1110 mL
D) It is not possible to make a more concentrated solution from a less concentrated solution.
A) 1.11 mL
B) 371 mL
C) 1110 mL
D) It is not possible to make a more concentrated solution from a less concentrated solution.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
75
A stock solution of 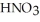 is prepared and found to contain 12.1 M of
is prepared and found to contain 12.1 M of 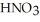 .If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L,the concentration of the diluted solution is ________ M.
.If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L,the concentration of the diluted solution is ________ M.
A) 0.242
B) 1.65
C) 0.605
D) 605
E) 242
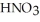 is prepared and found to contain 12.1 M of
is prepared and found to contain 12.1 M of 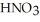 .If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L,the concentration of the diluted solution is ________ M.
.If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L,the concentration of the diluted solution is ________ M.A) 0.242
B) 1.65
C) 0.605
D) 605
E) 242

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
76
How many grams of 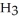 P
P 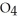 are in 325 mL of a 5.50 M solution of
are in 325 mL of a 5.50 M solution of 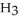 P
P 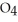 ?
?
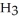 P
P 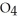 are in 325 mL of a 5.50 M solution of
are in 325 mL of a 5.50 M solution of 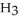 P
P 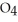 ?
?
Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
77
What is the concentration (M)of sodium ions in 4.57 L of a .533 M 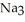 P solution?
P solution?
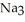 P solution?
P solution?
Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
78
Pure acetic acid ( 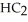
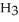
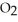 )is a liquid and is known as glacial acetic acid.Calculate the molarity of a solution prepared by dissolving 15.00 mL of glacial acetic acid at 25°C in sufficient water to give 500.0 mL of solution.The density of glacial acetic acid at 25°C is 1.05 g/mL.
)is a liquid and is known as glacial acetic acid.Calculate the molarity of a solution prepared by dissolving 15.00 mL of glacial acetic acid at 25°C in sufficient water to give 500.0 mL of solution.The density of glacial acetic acid at 25°C is 1.05 g/mL.
A) 1.89 × 103 M
B) 31.5 M
C) 0.0315 M
D) 0.525 M
E) 5.25 × 10-4 M
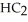
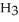
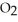 )is a liquid and is known as glacial acetic acid.Calculate the molarity of a solution prepared by dissolving 15.00 mL of glacial acetic acid at 25°C in sufficient water to give 500.0 mL of solution.The density of glacial acetic acid at 25°C is 1.05 g/mL.
)is a liquid and is known as glacial acetic acid.Calculate the molarity of a solution prepared by dissolving 15.00 mL of glacial acetic acid at 25°C in sufficient water to give 500.0 mL of solution.The density of glacial acetic acid at 25°C is 1.05 g/mL.A) 1.89 × 103 M
B) 31.5 M
C) 0.0315 M
D) 0.525 M
E) 5.25 × 10-4 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
79
What is the concentration of an AlCl3 solution if 150.mL of the solution contains 550.mg of Cl- ion?
A) 3.45 × 10-2 M
B) 8.25 × 10-2 M
C) 1.03 × 10-1 M
D) 3.10 × 10-1 M
A) 3.45 × 10-2 M
B) 8.25 × 10-2 M
C) 1.03 × 10-1 M
D) 3.10 × 10-1 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
80
A student prepared a stock solution by dissolving 15.0 g of KOH in enough water to make 150.mL of solution.She then took 15.0 mL of the stock solution and diluted it with enough water to make water to make 65.0 mL of a final solution.What is the concentration of KOH for the final solution?
A) 0. 411 M
B) 0. 534 M
C) 1.87 M
D) 2.43 M
A) 0. 411 M
B) 0. 534 M
C) 1.87 M
D) 2.43 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck


