Exam 9: Introduction to Solutions and Aqueous Reactions
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
Which of the following compounds is soluble in water?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
A
Determine the oxidation state of S in K2SO4.
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
D
Which of the following compounds is an Arrhenius base?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
D
How many milliliters of 0.550 M hydriodic acid are needed to react with 25.00 mL of 0.217 M CsOH?
HI(aq)+ CsOH(aq)→ CsI(aq)+ H2O(l)
(Multiple Choice)
4.9/5  (40)
(40)
How many grams of NaOH (MW = 40.0)are there in 500.0 mL of a 0.175 M NaOH solution?
(Short Answer)
4.9/5  (37)
(37)
What is the concentration of NO3- ions in a solution prepared by dissolving 15.0 g of Ca(NO3)2 in enough water to produce 300.mL of solution?
(Multiple Choice)
4.8/5  (43)
(43)
How many sodium ions are present in 125.0 mL of 0.750 M Na3PO4 solution?
(Multiple Choice)
4.8/5  (45)
(45)
According to the following reaction,how many moles of Fe(OH)2 can form from 175.0 mL of 0.227 M LiOH solution? Assume that there is excess FeCl2.
FeCl2(aq)+ 2 LiOH(aq)→ Fe(OH)2(s)+ 2 LiCl(aq)
(Multiple Choice)
4.8/5  (42)
(42)
When 7.80 mL of 0.500 M AgNO3 is added to 6.25 mL of 0.300 M NH4Cl,how many grams of AgCl are formed?
AgNO3(aq)+ NH4Cl(aq)→ AgCl(s)+ NH4NO3(aq)
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following compounds will undergo a gas-evolving reaction in an aqueous solution?
(Multiple Choice)
4.8/5  (39)
(39)
A student prepared a stock solution by dissolving 15.0 g of KOH in enough water to make 150.mL of solution.She then took 15.0 mL of the stock solution and diluted it with enough water to make water to make 65.0 mL of a final solution.What is the concentration of KOH for the final solution?
(Multiple Choice)
4.9/5  (39)
(39)
Determine the concentration of a solution prepared by diluting 25.0 mL of a stock 0.188 M Sr(NO3)2 solution to 150.0 mL.
(Multiple Choice)
4.8/5  (40)
(40)
How many carbonate ions are present in 30.0 mL of 0.600 M H2CO3 solution?
(Multiple Choice)
4.8/5  (35)
(35)
What is the concentration (M)of C 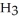 OH in a solution prepared by dissolving 3.96 g of C
OH in a solution prepared by dissolving 3.96 g of C 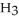 OH in sufficient water to give exactly 230 mL of solution?
OH in sufficient water to give exactly 230 mL of solution?
(Short Answer)
4.9/5  (43)
(43)
If the reaction of phosphate ion with water is ignored,what is the total concentration of ions in a solution prepared by dissolving 7.00 g of K3PO4 in enough water to make 350.mL of solution?
(Multiple Choice)
4.8/5  (33)
(33)
How many moles of Na I are required to make 250 mL of a 3.00 M solution?
(Multiple Choice)
4.8/5  (31)
(31)
How many grams of AgNO3 are needed to make 250.mL of a solution that is 0.155 M?
(Multiple Choice)
4.7/5  (39)
(39)
A stock solution of 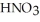 is prepared and found to contain 12.1 M of
is prepared and found to contain 12.1 M of 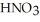 .If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L,the concentration of the diluted solution is ________ M.
.If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L,the concentration of the diluted solution is ________ M.
(Multiple Choice)
4.8/5  (38)
(38)
Showing 1 - 20 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)