Deck 14: Advanced Topics in Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 14: Advanced Topics in Equilibrium
1
A student is tasked to determine the pH of a solution prepared by dissolving 20.0 mmol sodium acetate,10.0 mmol sodium oxalate (NaO2CCO2H),and 15.0 mmol potassium chloride in enough water to prepare 1.00 L of solution.Which of the following will simplify the algebra used to solve the problem? I.Write fractional composition equations for each acid and base in the charge balance equation.
II Substitute the fractional composition equations into the charge balance equation and enter the known values of sodium,potassium,and chloride.
III Assume the weakest acid will not contribute significantly to the pH of the solution.
IV Use a spreadsheet to perform the calculations.
A)IV only
B)I,II,and III
C)I,II,III,and IV
D)II,III,and IV
E)I,II,and IV
II Substitute the fractional composition equations into the charge balance equation and enter the known values of sodium,potassium,and chloride.
III Assume the weakest acid will not contribute significantly to the pH of the solution.
IV Use a spreadsheet to perform the calculations.
A)IV only
B)I,II,and III
C)I,II,III,and IV
D)II,III,and IV
E)I,II,and IV
I,II,and IV
2
Calculate the concentration of each fumaric acid,HO2CCHCHCO2H,species in a 0.175 M solution at pH = 5.000.Ka1 = 9.5 × 10−4 and Ka2 = 3.3 × 10−5.
[H2A] = 0.000431 M,[HA−] = 0.0410 M and [A2−] = 0.1337 M.
3
The pH of a solution composed of NaO2CCO2H and NaCl must be determined.To get a more complete picture of the equilibrium,the mass balance equation for total oxalate is written.Which is the ONLY valid mass balance equation?
A)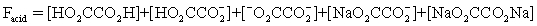
B)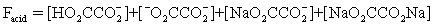
C)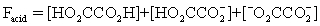
D)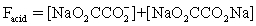
E)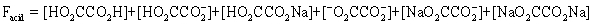
A)
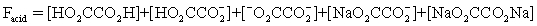
B)
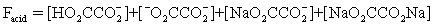
C)
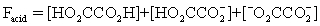
D)
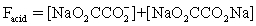
E)
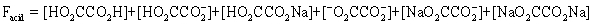
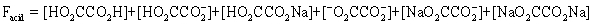
4
A solution is 0.200 M in acetic acid and 0.100 M KCl.Calculate the concentration acetic acid and acetate in solution for a pH 4.500. 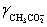 = 0.775,
= 0.775, 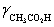 = 1,
= 1, 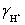 = 0.83 and Ka = 1.75 × 10−5.
= 0.83 and Ka = 1.75 × 10−5.
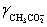 = 0.775,
= 0.775, 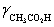 = 1,
= 1, 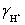 = 0.83 and Ka = 1.75 × 10−5.
= 0.83 and Ka = 1.75 × 10−5.
Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the fractional composition equations is incorrectly written for the diprotic acid H2A and the monoprotic acid HA?
A)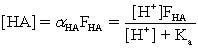
B)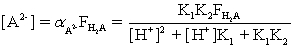
C)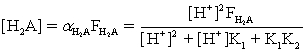
D)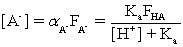
E)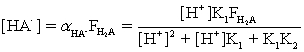
A)
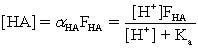
B)
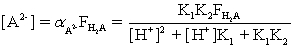
C)
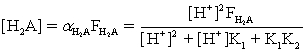
D)
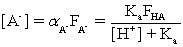
E)
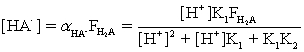

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
The mean fraction of protons,  ,bound to H3A ranges from____________________ and
,bound to H3A ranges from____________________ and 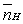 (theoretical)=____________________ .
(theoretical)=____________________ .
A)0 to 3;
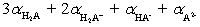
B)1 to 3;
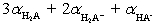
C)0 to 3;
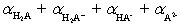
D)1 to 2;
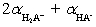
E)0 to 2;
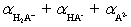
 ,bound to H3A ranges from____________________ and
,bound to H3A ranges from____________________ and 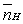 (theoretical)=____________________ .
(theoretical)=____________________ .A)0 to 3;
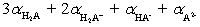
B)1 to 3;
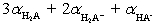
C)0 to 3;
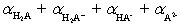
D)1 to 2;
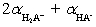
E)0 to 2;
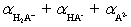

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
The pH of a solution composed of NaCH3CO2,NaO2CCO2H,and KCl must be determined.To get a more complete picture of the equilibrium,the ion-pairing equilibria are included in the calculations.Which of the following is NOT valid for ion pairing?
A)K+ + CH3CO2− ⇋ KCH3CO2
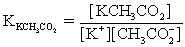
B)Na+ + -O2CCO2− ⇋ NaO2CCO2−
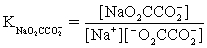
C)Na+ + Cl− ⇋ NaCl
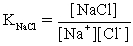
D)Na+ + CH3CO2− ⇋ NaCH3CO2
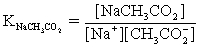
E)CH3CO2− + Cl− ⇋ CH3CO2Cl2−
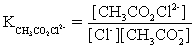
A)K+ + CH3CO2− ⇋ KCH3CO2
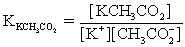
B)Na+ + -O2CCO2− ⇋ NaO2CCO2−
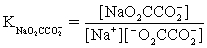
C)Na+ + Cl− ⇋ NaCl
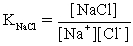
D)Na+ + CH3CO2− ⇋ NaCH3CO2
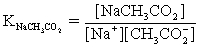
E)CH3CO2− + Cl− ⇋ CH3CO2Cl2−
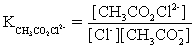

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
The mass balance equation for the solubility of copper(II)carbonate is [Cu2+] = [ ![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_2870_a4b1_b3052a98f71b_TB4000_11.jpg) ]
]
To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?
A)[Cu2+] + [CuOH+] + [CuCO3] = [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_2871_a4b1_236c9f3402b5_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_2872_a4b1_a7b7e8df1e46_TB4000_11.jpg)
]
B)[Cu2+] + [CuCO3] = [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_2873_a4b1_67097661ef64_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_2874_a4b1_a3029a29f49a_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_4f85_a4b1_c54f2fd4f4e1_TB4000_11.jpg)
]
C)[Cu2+] + [CuOH+] = [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_4f86_a4b1_b11c8213d8ef_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_4f87_a4b1_0388de112e10_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_4f88_a4b1_89265adf4717_TB4000_11.jpg)
] + [CuCO3]
D)[Cu2+] + [CuOH+] = [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_4f89_a4b1_b5051a17b4cc_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_4f8a_a4b1_0d2459c4b6bf_TB4000_11.jpg)
]+ [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_769b_a4b1_71d7fc2ccd56_TB4000_11.jpg)
]
E)[Cu2+] = [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_769c_a4b1_83480be89acd_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_769d_a4b1_0b1018c55837_TB4000_11.jpg)
]
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_2870_a4b1_b3052a98f71b_TB4000_11.jpg) ]
]To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?
A)[Cu2+] + [CuOH+] + [CuCO3] = [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_2871_a4b1_236c9f3402b5_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_2872_a4b1_a7b7e8df1e46_TB4000_11.jpg)
]
B)[Cu2+] + [CuCO3] = [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_2873_a4b1_67097661ef64_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_2874_a4b1_a3029a29f49a_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_4f85_a4b1_c54f2fd4f4e1_TB4000_11.jpg)
]
C)[Cu2+] + [CuOH+] = [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_4f86_a4b1_b11c8213d8ef_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_4f87_a4b1_0388de112e10_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_4f88_a4b1_89265adf4717_TB4000_11.jpg)
] + [CuCO3]
D)[Cu2+] + [CuOH+] = [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_4f89_a4b1_b5051a17b4cc_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_4f8a_a4b1_0d2459c4b6bf_TB4000_11.jpg)
]+ [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_769b_a4b1_71d7fc2ccd56_TB4000_11.jpg)
]
E)[Cu2+] = [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_769c_a4b1_83480be89acd_TB4000_11.jpg)
] + [
![<strong>The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?</strong> A)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] B)[Cu<sup>2+</sup>] + [CuCO<sub>3</sub>] = [ ] + [ ] + [ ] C)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ] + [ ] + [CuCO<sub>3</sub>] D)[Cu<sup>2+</sup>] + [CuOH<sup>+</sup>] = [ ] + [ ]+ [ ] E)[Cu<sup>2+</sup>] = [ ] + [ ]](https://storage.examlex.com/TB4000/11ea6952_25ab_769d_a4b1_0b1018c55837_TB4000_11.jpg)
]

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
Calculate the pH for a buffer that is 0.10 M in NaO2CCO2Na and 0.50 M in NaO2CCO2H.K2 = 5.42 × 10−5, 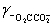 = 0.333,
= 0.333, 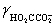 = 0.76
= 0.76
A)4)209
B)4)567
C)3)965
D)4)323
E)3)908
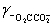 = 0.333,
= 0.333, 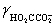 = 0.76
= 0.76A)4)209
B)4)567
C)3)965
D)4)323
E)3)908

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Which is the correct effective solubility constant expression for PbCl2? PbCl2 ⇋ Pb2+ + 2 Cl−
A)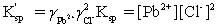
B)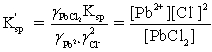
C)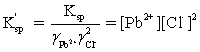
D)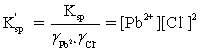
E)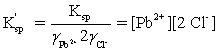
A)
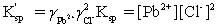
B)
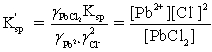
C)
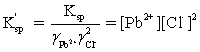
D)
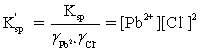
E)
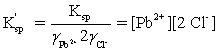

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Express [Ca2+] in terms [H+] for the solubility of CaSO4 using the equations below.Assume all activity coefficients are unity.
CaSO4 ⇋ Ca2+ +![Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the solubility of CaSO<sub>4</sub> using the equations below.Assume all activity coefficients are unity. CaSO<sub>4</sub> ⇋ Ca<sup>2+</sup> + K<sub>sp</sub> = 2.40 × 10<sup>−</sup><sup>5</sup> Ca<sup>2+</sup> + H<sub>2</sub>O ⇋ CaOH<sup>+</sup> + H<sup>+</sup>____________________K<sub>a</sub> = 2.00 × 10<sup>−</sup><sup>13</sup> Ca<sup>2+</sup> + ⇋ CaSO<sub>4</sub> K<sub>ip</sub> = 229 ⇋ H<sup>+</sup> + K<sub>a2</sub> = 1.03 × 10<sup>−</sup><sup>2</sup>](https://storage.examlex.com/TB4000/11ea6952_25ac_12ea_a4b1_4f08360b66de_TB4000_11.jpg) Ksp = 2.40 × 10−5
Ksp = 2.40 × 10−5
Ca2+ + H2O ⇋ CaOH+ + H+____________________Ka = 2.00 × 10−13
Ca2+ +![Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the solubility of CaSO<sub>4</sub> using the equations below.Assume all activity coefficients are unity. CaSO<sub>4</sub> ⇋ Ca<sup>2+</sup> + K<sub>sp</sub> = 2.40 × 10<sup>−</sup><sup>5</sup> Ca<sup>2+</sup> + H<sub>2</sub>O ⇋ CaOH<sup>+</sup> + H<sup>+</sup>____________________K<sub>a</sub> = 2.00 × 10<sup>−</sup><sup>13</sup> Ca<sup>2+</sup> + ⇋ CaSO<sub>4</sub> K<sub>ip</sub> = 229 ⇋ H<sup>+</sup> + K<sub>a2</sub> = 1.03 × 10<sup>−</sup><sup>2</sup>](https://storage.examlex.com/TB4000/11ea6952_25ac_39fb_a4b1_87be838cb12a_TB4000_11.jpg) ⇋ CaSO4 Kip = 229
⇋ CaSO4 Kip = 229 ![Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the solubility of CaSO<sub>4</sub> using the equations below.Assume all activity coefficients are unity. CaSO<sub>4</sub> ⇋ Ca<sup>2+</sup> + K<sub>sp</sub> = 2.40 × 10<sup>−</sup><sup>5</sup> Ca<sup>2+</sup> + H<sub>2</sub>O ⇋ CaOH<sup>+</sup> + H<sup>+</sup>____________________K<sub>a</sub> = 2.00 × 10<sup>−</sup><sup>13</sup> Ca<sup>2+</sup> + ⇋ CaSO<sub>4</sub> K<sub>ip</sub> = 229 ⇋ H<sup>+</sup> + K<sub>a2</sub> = 1.03 × 10<sup>−</sup><sup>2</sup>](https://storage.examlex.com/TB4000/11ea6952_25ac_39fc_a4b1_2701fcea5d6f_TB4000_11.jpg) ⇋ H+ +
⇋ H+ + ![Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the solubility of CaSO<sub>4</sub> using the equations below.Assume all activity coefficients are unity. CaSO<sub>4</sub> ⇋ Ca<sup>2+</sup> + K<sub>sp</sub> = 2.40 × 10<sup>−</sup><sup>5</sup> Ca<sup>2+</sup> + H<sub>2</sub>O ⇋ CaOH<sup>+</sup> + H<sup>+</sup>____________________K<sub>a</sub> = 2.00 × 10<sup>−</sup><sup>13</sup> Ca<sup>2+</sup> + ⇋ CaSO<sub>4</sub> K<sub>ip</sub> = 229 ⇋ H<sup>+</sup> + K<sub>a2</sub> = 1.03 × 10<sup>−</sup><sup>2</sup>](https://storage.examlex.com/TB4000/11ea6952_25ac_39fd_a4b1_d779495a768f_TB4000_11.jpg) Ka2 = 1.03 × 10−2
Ka2 = 1.03 × 10−2
CaSO4 ⇋ Ca2+ +
![Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the solubility of CaSO<sub>4</sub> using the equations below.Assume all activity coefficients are unity. CaSO<sub>4</sub> ⇋ Ca<sup>2+</sup> + K<sub>sp</sub> = 2.40 × 10<sup>−</sup><sup>5</sup> Ca<sup>2+</sup> + H<sub>2</sub>O ⇋ CaOH<sup>+</sup> + H<sup>+</sup>____________________K<sub>a</sub> = 2.00 × 10<sup>−</sup><sup>13</sup> Ca<sup>2+</sup> + ⇋ CaSO<sub>4</sub> K<sub>ip</sub> = 229 ⇋ H<sup>+</sup> + K<sub>a2</sub> = 1.03 × 10<sup>−</sup><sup>2</sup>](https://storage.examlex.com/TB4000/11ea6952_25ac_12ea_a4b1_4f08360b66de_TB4000_11.jpg) Ksp = 2.40 × 10−5
Ksp = 2.40 × 10−5Ca2+ + H2O ⇋ CaOH+ + H+____________________Ka = 2.00 × 10−13
Ca2+ +
![Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the solubility of CaSO<sub>4</sub> using the equations below.Assume all activity coefficients are unity. CaSO<sub>4</sub> ⇋ Ca<sup>2+</sup> + K<sub>sp</sub> = 2.40 × 10<sup>−</sup><sup>5</sup> Ca<sup>2+</sup> + H<sub>2</sub>O ⇋ CaOH<sup>+</sup> + H<sup>+</sup>____________________K<sub>a</sub> = 2.00 × 10<sup>−</sup><sup>13</sup> Ca<sup>2+</sup> + ⇋ CaSO<sub>4</sub> K<sub>ip</sub> = 229 ⇋ H<sup>+</sup> + K<sub>a2</sub> = 1.03 × 10<sup>−</sup><sup>2</sup>](https://storage.examlex.com/TB4000/11ea6952_25ac_39fb_a4b1_87be838cb12a_TB4000_11.jpg) ⇋ CaSO4 Kip = 229
⇋ CaSO4 Kip = 229 ![Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the solubility of CaSO<sub>4</sub> using the equations below.Assume all activity coefficients are unity. CaSO<sub>4</sub> ⇋ Ca<sup>2+</sup> + K<sub>sp</sub> = 2.40 × 10<sup>−</sup><sup>5</sup> Ca<sup>2+</sup> + H<sub>2</sub>O ⇋ CaOH<sup>+</sup> + H<sup>+</sup>____________________K<sub>a</sub> = 2.00 × 10<sup>−</sup><sup>13</sup> Ca<sup>2+</sup> + ⇋ CaSO<sub>4</sub> K<sub>ip</sub> = 229 ⇋ H<sup>+</sup> + K<sub>a2</sub> = 1.03 × 10<sup>−</sup><sup>2</sup>](https://storage.examlex.com/TB4000/11ea6952_25ac_39fc_a4b1_2701fcea5d6f_TB4000_11.jpg) ⇋ H+ +
⇋ H+ + ![Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the solubility of CaSO<sub>4</sub> using the equations below.Assume all activity coefficients are unity. CaSO<sub>4</sub> ⇋ Ca<sup>2+</sup> + K<sub>sp</sub> = 2.40 × 10<sup>−</sup><sup>5</sup> Ca<sup>2+</sup> + H<sub>2</sub>O ⇋ CaOH<sup>+</sup> + H<sup>+</sup>____________________K<sub>a</sub> = 2.00 × 10<sup>−</sup><sup>13</sup> Ca<sup>2+</sup> + ⇋ CaSO<sub>4</sub> K<sub>ip</sub> = 229 ⇋ H<sup>+</sup> + K<sub>a2</sub> = 1.03 × 10<sup>−</sup><sup>2</sup>](https://storage.examlex.com/TB4000/11ea6952_25ac_39fd_a4b1_d779495a768f_TB4000_11.jpg) Ka2 = 1.03 × 10−2
Ka2 = 1.03 × 10−2
Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
A solution is 0.120 M sodium oxalate (NaO2CCO2H),0.05 M chloroacetic acid (ClCH2CO2H)and 0.01 M KOH.Express the charge balance equation for the solution in terms of [H+].

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
In addition to the equilibrium CaCO3 ⇋ Ca2+ + CO32-,additional equilibria can be written to give a more complete picture of calcium carbonate's solubility.Which of the following is NOT valid?
A)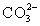
+ H2O ⇋
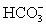
+ OH-
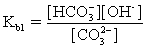
B)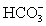
+ H2O ⇋ H2CO3 + OH-
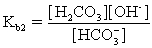
C)Ca2+ + H2O ⇋ CaOH+ + H+
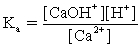
D)Ca2+ +
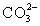
⇋ CaCO3
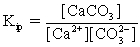
E)Ca2+ + H+ ⇋ CaH3+
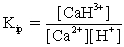
A)
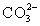
+ H2O ⇋
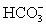
+ OH-
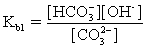
B)
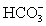
+ H2O ⇋ H2CO3 + OH-
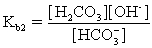
C)Ca2+ + H2O ⇋ CaOH+ + H+
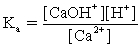
D)Ca2+ +
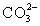
⇋ CaCO3
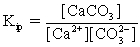
E)Ca2+ + H+ ⇋ CaH3+
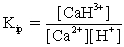

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
The rearrangement of the equilibrium constant expression to incorporate activity coefficients at the given ionic strength is the:
A)conditional equilibrium constant,K'.
B)temporary equilibrium constant,K'.
C)combined equilibrium constant,K'.
D)effective equilibrium constant,K'.
E)activity equilibrium constant,K'.
A)conditional equilibrium constant,K'.
B)temporary equilibrium constant,K'.
C)combined equilibrium constant,K'.
D)effective equilibrium constant,K'.
E)activity equilibrium constant,K'.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
Calculate the pH of a sulfurous acid buffer prepared from 0.010 M NaHSO3 and 0.030 M Na2SO3.Ka1 = 1.39 × 10−2 and Ka2 = 6.73 × 10−8 for sulfurous acid.
Ionic strength (,M)
Ion
0.001
0.005
0.01
0.05
0.1
Charge = 1
Activity coefficient ()
Na+,0.964
0.928
0.902
0.82
0.775
Charge = 2
Activity coefficient ()0.867
0.742
0.665
0.455
0.37
Ionic strength (,M)
Ion
0.001
0.005
0.01
0.05
0.1
Charge = 1
Activity coefficient ()
Na+,0.964
0.928
0.902
0.82
0.775
Charge = 2
Activity coefficient ()0.867
0.742
0.665
0.455
0.37

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
The charge balance equation for the solubility of cobalt sulfide is [H+] + 2 [Co2+] = 2 [S2−] + [OH−]
To determine a more accurate solubility for cobalt sulfide,additional equilibria must be written for cobalt(II)and sulfide in solution.Which of the charge balance equations below is the only valid charge balance equation?
A)[H+] + 2 [Co2+] + [CoOH+] + 3 [CoH3+] = 2 [S2−] + [HS−] + [OH−]
B)[H+] + 2 [Co2+] + [CoOH+] = 2 [S2−] + [HS−] + [OH−] + 3 [SOH3−]
C)[H+] + 2 [Co2+] + [CoOH+] = 2 [S2−] + [HS−] + [OH−]
D)[H+] + 2 [Co2+] + 3 [CoH3+] = 2 [S2−] + [HS−] + [OH−] + 3 [SOH3−]
E)[H+] + 2 [Co2+] + [CoOH+] + 3 [CoH3+] = 2 [S2−] + [HS−] + [OH−] + 3 [SOH3−]
To determine a more accurate solubility for cobalt sulfide,additional equilibria must be written for cobalt(II)and sulfide in solution.Which of the charge balance equations below is the only valid charge balance equation?
A)[H+] + 2 [Co2+] + [CoOH+] + 3 [CoH3+] = 2 [S2−] + [HS−] + [OH−]
B)[H+] + 2 [Co2+] + [CoOH+] = 2 [S2−] + [HS−] + [OH−] + 3 [SOH3−]
C)[H+] + 2 [Co2+] + [CoOH+] = 2 [S2−] + [HS−] + [OH−]
D)[H+] + 2 [Co2+] + 3 [CoH3+] = 2 [S2−] + [HS−] + [OH−] + 3 [SOH3−]
E)[H+] + 2 [Co2+] + [CoOH+] + 3 [CoH3+] = 2 [S2−] + [HS−] + [OH−] + 3 [SOH3−]

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
The equilibrium constants for a diprotic acid are determined from a Bjerrum plot by:
A)fitting the theoretical curve to the experimental curve by adjusting the pH values to minimize the square of the residuals.
B)determining the slope for the upper curve which corresponds to K1,and the slope for the lower curve which corresponds to K2.
C)varying the pH to determine the range over which the slope of the theoretical curve is zero.Lower end of range is K1 and upper end of range is K2.
D)fitting the theoretical curve to the experimental curve by the method of least squares to find the values of K1 and K2 that minimize the square of the residuals.
E)taking the first derivative of the experimental data to determine minimums in slope.Each minimum is an equilibrium constant.
A)fitting the theoretical curve to the experimental curve by adjusting the pH values to minimize the square of the residuals.
B)determining the slope for the upper curve which corresponds to K1,and the slope for the lower curve which corresponds to K2.
C)varying the pH to determine the range over which the slope of the theoretical curve is zero.Lower end of range is K1 and upper end of range is K2.
D)fitting the theoretical curve to the experimental curve by the method of least squares to find the values of K1 and K2 that minimize the square of the residuals.
E)taking the first derivative of the experimental data to determine minimums in slope.Each minimum is an equilibrium constant.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
Which is the correct effective equilibrium constant expression for the reaction of NaO2CCO2H with water? 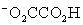 ⇋
⇋ 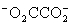 + H+
+ H+
A)
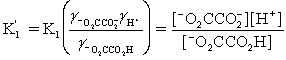
B)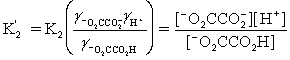
C)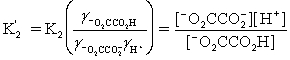
D)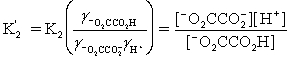
E)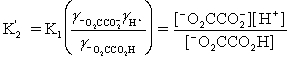
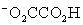 ⇋
⇋ 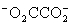 + H+
+ H+A)
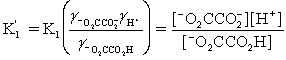
B)
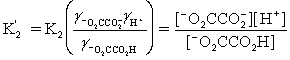
C)
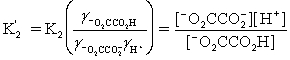
D)
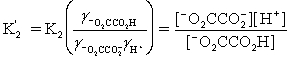
E)
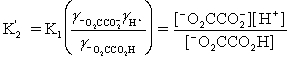

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate the activity coefficient for phosphate in 0.0075 M KCl. 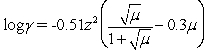
A)0)441
B)0)140
C)0)804
D)0)913
E)0)116
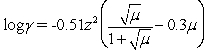
A)0)441
B)0)140
C)0)804
D)0)913
E)0)116

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
Many ions do not have tabulated activity coefficients nor can the activity coefficient be estimated,as the ion size is now known.The Davies equation is used to estimate the activity coefficient under these conditions.Which statement(s)is(are)NOT true for the Davies equation?
I The calculated activity coefficient is independent of the ion's size.
II The ion's charge,the solutions ionic strength and the ion size are required to calculate the activity coefficient.
III The Davies equation can be used up to ~ 0.5 M ionic strength.
IV The calculated activity coefficient is no more accurate than guessing the ion size to determine the activity coefficient.
A)II and IV
B)II
C)I
D)III and IV
E)III
I The calculated activity coefficient is independent of the ion's size.
II The ion's charge,the solutions ionic strength and the ion size are required to calculate the activity coefficient.
III The Davies equation can be used up to ~ 0.5 M ionic strength.
IV The calculated activity coefficient is no more accurate than guessing the ion size to determine the activity coefficient.
A)II and IV
B)II
C)I
D)III and IV
E)III

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


