Exam 14: Advanced Topics in Equilibrium
Exam 1: The Analytical Process20 Questions
Exam 2: Chemical Measurements21 Questions
Exam 3: Tools of the Trade19 Questions
Exam 4: Experimental Error20 Questions
Exam 5: Statistics22 Questions
Exam 6: Quality Assurance and Calibration Methods20 Questions
Exam 7: Chemical Equilibrium20 Questions
Exam 8: Let the Titrations Begin20 Questions
Exam 9: Activity and the Systematic Treatment of Equilibrium20 Questions
Exam 10: Monoprotic Acid-Base Equilibria20 Questions
Exam 11: Polyprotic Acid-Base Equilibria20 Questions
Exam 12: Acid-Base Titrations20 Questions
Exam 13: Edta Titrations20 Questions
Exam 14: Advanced Topics in Equilibrium20 Questions
Exam 15: Fundamentals of Electrochemistry20 Questions
Exam 16: Electrodes and Potentiometry20 Questions
Exam 17: Redox Titrations20 Questions
Exam 18: Electroanalytical Techniques20 Questions
Exam 19: Fundamentals of Spectrophotometry23 Questions
Exam 20: Applications of Spectrophotometry20 Questions
Exam 21: Spectrophotometers20 Questions
Exam 22: Atomic Spectroscopy20 Questions
Exam 23: Mass Spectrometry20 Questions
Exam 24: Introduction to Analytical Separations20 Questions
Exam 25: Gas Chromatography20 Questions
Exam 26: High-Performance Liquid Chromatography20 Questions
Exam 27: Chromatographic Methods and Capillary Electrophoresis20 Questions
Exam 28: Gravimetric and Combustion Analysis20 Questions
Exam 29: Sample Preparation19 Questions
Select questions type
The charge balance equation for the solubility of cobalt sulfide is [H+] + 2 [Co2+] = 2 [S2−] + [OH−]
To determine a more accurate solubility for cobalt sulfide,additional equilibria must be written for cobalt(II)and sulfide in solution.Which of the charge balance equations below is the only valid charge balance equation?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
C
Which is the correct effective equilibrium constant expression for the reaction of NaO2CCO2H with water? 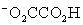 ⇋
⇋ 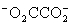 + H+
+ H+
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
D
Many ions do not have tabulated activity coefficients nor can the activity coefficient be estimated,as the ion size is now known.The Davies equation is used to estimate the activity coefficient under these conditions.Which statement(s)is(are)NOT true for the Davies equation?
I The calculated activity coefficient is independent of the ion's size.
II The ion's charge,the solutions ionic strength and the ion size are required to calculate the activity coefficient.
III The Davies equation can be used up to ~ 0.5 M ionic strength.
IV The calculated activity coefficient is no more accurate than guessing the ion size to determine the activity coefficient.
Free
(Multiple Choice)
5.0/5  (29)
(29)
Correct Answer:
B
A student is tasked to determine the pH of a solution prepared by dissolving 20.0 mmol sodium acetate,10.0 mmol sodium oxalate (NaO2CCO2H),and 15.0 mmol potassium chloride in enough water to prepare 1.00 L of solution.Which of the following will simplify the algebra used to solve the problem? I.Write fractional composition equations for each acid and base in the charge balance equation.
II Substitute the fractional composition equations into the charge balance equation and enter the known values of sodium,potassium,and chloride.
III Assume the weakest acid will not contribute significantly to the pH of the solution.
IV Use a spreadsheet to perform the calculations.
(Multiple Choice)
4.9/5  (43)
(43)
Calculate the activity coefficient for phosphate in 0.0075 M KCl. 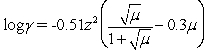
(Multiple Choice)
4.8/5  (25)
(25)
Calculate the pH of a sulfurous acid buffer prepared from 0.010 M NaHSO3 and 0.030 M Na2SO3.Ka1 = 1.39 × 10−2 and Ka2 = 6.73 × 10−8 for sulfurous acid.
Ionic strength (,M)
Ion
0.001
0.005
0.01
0.05
0.1
Charge = 1
Activity coefficient ()
Na+,0.964
0.928
0.902
0.82
0.775
Charge = 2
Activity coefficient ()0.867
0.742
0.665
0.455
0.37
(Essay)
4.7/5  (25)
(25)
The rearrangement of the equilibrium constant expression to incorporate activity coefficients at the given ionic strength is the:
(Multiple Choice)
4.8/5  (40)
(40)
The mean fraction of protons,  ,bound to H3A ranges from____________________ and
,bound to H3A ranges from____________________ and 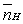 (theoretical)=____________________ .
(theoretical)=____________________ .
(Multiple Choice)
4.7/5  (41)
(41)
A solution is 0.120 M sodium oxalate (NaO2CCO2H),0.05 M chloroacetic acid (ClCH2CO2H)and 0.01 M KOH.Express the charge balance equation for the solution in terms of [H+].
(Essay)
4.8/5  (41)
(41)
The equilibrium constants for a diprotic acid are determined from a Bjerrum plot by:
(Multiple Choice)
4.9/5  (37)
(37)
A solution is 0.200 M in acetic acid and 0.100 M KCl.Calculate the concentration acetic acid and acetate in solution for a pH 4.500. 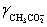 = 0.775,
= 0.775, 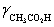 = 1,
= 1, 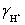 = 0.83 and Ka = 1.75 × 10−5.
= 0.83 and Ka = 1.75 × 10−5.
(Essay)
5.0/5  (39)
(39)
Calculate the pH for a buffer that is 0.10 M in NaO2CCO2Na and 0.50 M in NaO2CCO2H.K2 = 5.42 × 10−5, 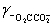 = 0.333,
= 0.333, 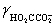 = 0.76
= 0.76
(Multiple Choice)
4.9/5  (33)
(33)
The pH of a solution composed of NaCH3CO2,NaO2CCO2H,and KCl must be determined.To get a more complete picture of the equilibrium,the ion-pairing equilibria are included in the calculations.Which of the following is NOT valid for ion pairing?
(Multiple Choice)
4.9/5  (41)
(41)
Calculate the concentration of each fumaric acid,HO2CCHCHCO2H,species in a 0.175 M solution at pH = 5.000.Ka1 = 9.5 × 10−4 and Ka2 = 3.3 × 10−5.
(Essay)
4.9/5  (40)
(40)
Express [Ca2+] in terms [H+] for the solubility of CaSO4 using the equations below.Assume all activity coefficients are unity.
CaSO4 ⇋ Ca2+ + ![Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the solubility of CaSO<sub>4</sub> using the equations below.Assume all activity coefficients are unity. CaSO<sub>4</sub> ⇋ Ca<sup>2+</sup> + K<sub>sp</sub> = 2.40 × 10<sup>−</sup><sup>5</sup> Ca<sup>2+</sup> + H<sub>2</sub>O ⇋ CaOH<sup>+</sup> + H<sup>+</sup>____________________K<sub>a</sub> = 2.00 × 10<sup>−</sup><sup>13</sup> Ca<sup>2+</sup> + ⇋ CaSO<sub>4</sub> K<sub>ip</sub> = 229 ⇋ H<sup>+</sup> + K<sub>a2</sub> = 1.03 × 10<sup>−</sup><sup>2</sup>](https://storage.examlex.com/TB4000/11ea6952_25ac_12ea_a4b1_4f08360b66de_TB4000_11.jpg) Ksp = 2.40 × 10−5
Ca2+ + H2O ⇋ CaOH+ + H+____________________Ka = 2.00 × 10−13
Ca2+ +
Ksp = 2.40 × 10−5
Ca2+ + H2O ⇋ CaOH+ + H+____________________Ka = 2.00 × 10−13
Ca2+ + ![Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the solubility of CaSO<sub>4</sub> using the equations below.Assume all activity coefficients are unity. CaSO<sub>4</sub> ⇋ Ca<sup>2+</sup> + K<sub>sp</sub> = 2.40 × 10<sup>−</sup><sup>5</sup> Ca<sup>2+</sup> + H<sub>2</sub>O ⇋ CaOH<sup>+</sup> + H<sup>+</sup>____________________K<sub>a</sub> = 2.00 × 10<sup>−</sup><sup>13</sup> Ca<sup>2+</sup> + ⇋ CaSO<sub>4</sub> K<sub>ip</sub> = 229 ⇋ H<sup>+</sup> + K<sub>a2</sub> = 1.03 × 10<sup>−</sup><sup>2</sup>](https://storage.examlex.com/TB4000/11ea6952_25ac_39fb_a4b1_87be838cb12a_TB4000_11.jpg) ⇋ CaSO4 Kip = 229
⇋ CaSO4 Kip = 229 ![Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the solubility of CaSO<sub>4</sub> using the equations below.Assume all activity coefficients are unity. CaSO<sub>4</sub> ⇋ Ca<sup>2+</sup> + K<sub>sp</sub> = 2.40 × 10<sup>−</sup><sup>5</sup> Ca<sup>2+</sup> + H<sub>2</sub>O ⇋ CaOH<sup>+</sup> + H<sup>+</sup>____________________K<sub>a</sub> = 2.00 × 10<sup>−</sup><sup>13</sup> Ca<sup>2+</sup> + ⇋ CaSO<sub>4</sub> K<sub>ip</sub> = 229 ⇋ H<sup>+</sup> + K<sub>a2</sub> = 1.03 × 10<sup>−</sup><sup>2</sup>](https://storage.examlex.com/TB4000/11ea6952_25ac_39fc_a4b1_2701fcea5d6f_TB4000_11.jpg) ⇋ H+ +
⇋ H+ + ![Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the solubility of CaSO<sub>4</sub> using the equations below.Assume all activity coefficients are unity. CaSO<sub>4</sub> ⇋ Ca<sup>2+</sup> + K<sub>sp</sub> = 2.40 × 10<sup>−</sup><sup>5</sup> Ca<sup>2+</sup> + H<sub>2</sub>O ⇋ CaOH<sup>+</sup> + H<sup>+</sup>____________________K<sub>a</sub> = 2.00 × 10<sup>−</sup><sup>13</sup> Ca<sup>2+</sup> + ⇋ CaSO<sub>4</sub> K<sub>ip</sub> = 229 ⇋ H<sup>+</sup> + K<sub>a2</sub> = 1.03 × 10<sup>−</sup><sup>2</sup>](https://storage.examlex.com/TB4000/11ea6952_25ac_39fd_a4b1_d779495a768f_TB4000_11.jpg) Ka2 = 1.03 × 10−2
Ka2 = 1.03 × 10−2
(Short Answer)
4.7/5  (29)
(29)
In addition to the equilibrium CaCO3 ⇋ Ca2+ + CO32-,additional equilibria can be written to give a more complete picture of calcium carbonate's solubility.Which of the following is NOT valid?
(Multiple Choice)
4.9/5  (35)
(35)
The mass balance equation for the solubility of copper(II)carbonate is [Cu2+] = [ ![The mass balance equation for the solubility of copper(II)carbonate is [Cu<sup>2+</sup>] = [ ] To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?](https://storage.examlex.com/TB4000/11ea6952_25ab_2870_a4b1_b3052a98f71b_TB4000_11.jpg) ]
To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?
]
To determine a more accurate solubility for copper(II)carbonate,additional equilibria must be written for copper(II)and carbonate in solution.Which of the mass balance equations below is the only valid charge balance equation?
(Multiple Choice)
4.8/5  (34)
(34)
Which is the correct effective solubility constant expression for PbCl2? PbCl2 ⇋ Pb2+ + 2 Cl−
(Multiple Choice)
4.9/5  (44)
(44)
The pH of a solution composed of NaO2CCO2H and NaCl must be determined.To get a more complete picture of the equilibrium,the mass balance equation for total oxalate is written.Which is the ONLY valid mass balance equation?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the fractional composition equations is incorrectly written for the diprotic acid H2A and the monoprotic acid HA?
(Multiple Choice)
4.8/5  (29)
(29)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)