Deck 15: Fundamentals of Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 15: Fundamentals of Electrochemistry
1
For the line notation below,which is NOT true? Fe(s)| Fe2+ (aq,A = 1)|| Cu2+ (aq,A = 1)| Cu (s)
A)Fe is the anode.
B)|| represents the salt bridge.
C)Cu is the anode.
D)1 M Cu2+ is the electrolyte concentration and species for the copper electrode.
E)1 M Fe2+ is the electrolyte concentration and species for the iron electrode.
A)Fe is the anode.
B)|| represents the salt bridge.
C)Cu is the anode.
D)1 M Cu2+ is the electrolyte concentration and species for the copper electrode.
E)1 M Fe2+ is the electrolyte concentration and species for the iron electrode.
Cu is the anode.
2
Calculate the equilibrium constant for the reaction between Sn metal and Zn2+ solution. Sn + Zn2+ ⇋ Sn2+ + Zn
Sn2+ + 2 e− → Sn (s)E° = −0.141 V
Zn2+ + 2 e− → Zn (s)E° = −0.762 V
A)9)86 × 1020
B)1)21 × 10−21
C)3)37 × 1030
D)3)18 × 10−11
E)1)84 × 1015
Sn2+ + 2 e− → Sn (s)E° = −0.141 V
Zn2+ + 2 e− → Zn (s)E° = −0.762 V
A)9)86 × 1020
B)1)21 × 10−21
C)3)37 × 1030
D)3)18 × 10−11
E)1)84 × 1015
1)21 × 10−21
3
Calculate E° for the half-cell below. O2 + 4 H+ + 4 e− ⇋ 2 H2O E° = 1.229 V
A)1)643 V
B)0)815 V
C)0)307 V
D)1)229 V
E)0)711 V
A)1)643 V
B)0)815 V
C)0)307 V
D)1)229 V
E)0)711 V
0)815 V
4
For the silver half-reaction,Ag+ + 1 e- → Ag (s),when the concentration of silver cation is increased,the reduction potential:
A)becomes more positive.
B)becomes more negative.
C)remains constant.
D)increases or decreases depending on the voltage of the other half-reaction.
E)increases or decreases depending on the temperature.
A)becomes more positive.
B)becomes more negative.
C)remains constant.
D)increases or decreases depending on the voltage of the other half-reaction.
E)increases or decreases depending on the temperature.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
Calculate Eo and K for the cell composed of a bromate/bromide half-cell and a chlorate/chlorine dioxide half-cell. 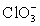 + 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V
+ 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V 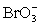 + 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
+ 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
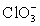 + 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V
+ 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V 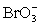 + 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
+ 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
For the cell diagram below write out and balance the reaction that occurs in the cell.
Pt|HOCl (aq,1 M),HClO2 (aq,1 M),H+ (aq,1 M)||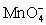 (aq,1 M),H+ (aq,1 M)| MnO2 (s)|Pt
(aq,1 M),H+ (aq,1 M)| MnO2 (s)|Pt
Pt|HOCl (aq,1 M),HClO2 (aq,1 M),H+ (aq,1 M)||
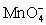 (aq,1 M),H+ (aq,1 M)| MnO2 (s)|Pt
(aq,1 M),H+ (aq,1 M)| MnO2 (s)|Pt
Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
All of the following are true for the measurement of standard reduction potentials,except:
I standard conditions for reduction potentials is 25 oC and A = 1 for all aqueous and gaseous species.
II reduction potentials are measured relative to the standard hydrogen electrode (S.H.E. ).
III standard reduction potentials are measured while connected to the negative electrode of the potentiometer.
IV the E° for the S.H.E.is defined as 0 volts.
A)I and IV
B)I and III
C)I only
D)III only
E)II and IV
I standard conditions for reduction potentials is 25 oC and A = 1 for all aqueous and gaseous species.
II reduction potentials are measured relative to the standard hydrogen electrode (S.H.E. ).
III standard reduction potentials are measured while connected to the negative electrode of the potentiometer.
IV the E° for the S.H.E.is defined as 0 volts.
A)I and IV
B)I and III
C)I only
D)III only
E)II and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
Calculate the standard potential when the two half-cells below are connected via a salt bridge and potentiometer.Which species are the reducing agent and oxidizing agent? 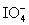 + 2 H+ + 2 e- ⇋
+ 2 H+ + 2 e- ⇋ 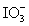 + H2O E° = 1.589 V
+ H2O E° = 1.589 V
Mg(OH)2 (s)+ 2 e- ⇋ Mg (s)+ 2 OH- E° = -2.690 V
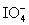 + 2 H+ + 2 e- ⇋
+ 2 H+ + 2 e- ⇋ 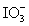 + H2O E° = 1.589 V
+ H2O E° = 1.589 VMg(OH)2 (s)+ 2 e- ⇋ Mg (s)+ 2 OH- E° = -2.690 V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
A bromide probe is constructed from a saturated silver bromide half-cell and a Ni2+/Ni half-cell.If the measured potential is 0.952 V and [Ni2+] = 1 M,what is the bromide concentration?
AgBr (s)+ e− ⇋ Ag(s)+ Br− E° = 0.071 V
Ni2+ + 2 e− ⇋ Ni (s)E° = −0.714 V
AgBr (s)+ e− ⇋ Ag(s)+ Br− E° = 0.071 V
Ni2+ + 2 e− ⇋ Ni (s)E° = −0.714 V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
For the reaction below,the oxidizing agent is____________________ and it____________________ electrons,and the reducing agent is____________________ and it____________________ electrons. 8 H+ + 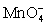 + 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2O
+ 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2O
A)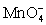
;gains 5;Cu+;loses 1
B)H+;loses 1;Cu+;gains 1
C)H+;gains 1;
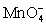
;loses 5
D)Cu+;loses 1;
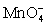
;gains 1
E)Cu+;loses 1;H+;gains 1
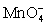 + 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2O
+ 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2OA)
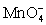
;gains 5;Cu+;loses 1
B)H+;loses 1;Cu+;gains 1
C)H+;gains 1;
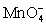
;loses 5
D)Cu+;loses 1;
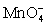
;gains 1
E)Cu+;loses 1;H+;gains 1

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
A galvanic cell is at equilibrium when:
A)Ecell is a constant value.
B)cell resistance is very low.
C)Ecell = 0 V.
D)Ecell fluctuates between E°cell.
E)cell resistance is infinitely large.
A)Ecell is a constant value.
B)cell resistance is very low.
C)Ecell = 0 V.
D)Ecell fluctuates between E°cell.
E)cell resistance is infinitely large.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
Which is true when electrochemical cells are used as chemical probes?
I.All concentrations in the half-cells must be known except for the analyte of interest.
II. The analyte concentration is calculated from the equilibrium cell potential.
III. Equilibrium between the half-cells is established.
IV. Equilibrium within each half-cell is established and is assumed to remain at equilibrium.
V. Half-cells other than the S.H.E.can be used to determine pH.
A)II,III,and IV
B)I,IV,and V
C)I and II
D)III,IV,and V
E)I,II,and V
I.All concentrations in the half-cells must be known except for the analyte of interest.
II. The analyte concentration is calculated from the equilibrium cell potential.
III. Equilibrium between the half-cells is established.
IV. Equilibrium within each half-cell is established and is assumed to remain at equilibrium.
V. Half-cells other than the S.H.E.can be used to determine pH.
A)II,III,and IV
B)I,IV,and V
C)I and II
D)III,IV,and V
E)I,II,and V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Which is NOT correct for when the silver and vanadium half-cells are connected via a salt bridge and a potentiometer?
Ag+ + 1 e- → Ag (s)E° = 0.7993 V
V2+ + 2 e- → V (s)E° = −1.125 V
I Ag+ is reduced.
II V is oxidized.
III Eocell = 1.924 V
IV V2+ is reduced.
V Ag is oxidized.
A)I and II
B)III,IV,and V
C)I,II,and III
D)III only
E)IV and V
Ag+ + 1 e- → Ag (s)E° = 0.7993 V
V2+ + 2 e- → V (s)E° = −1.125 V
I Ag+ is reduced.
II V is oxidized.
III Eocell = 1.924 V
IV V2+ is reduced.
V Ag is oxidized.
A)I and II
B)III,IV,and V
C)I,II,and III
D)III only
E)IV and V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Eo is defined as:
A)the formal half-cell potential for compounds that are neither acids nor bases.
B)the formal half-cell potential at 20 C.
C)the formal half-cell potential at pH 7.
D)the inverse of E°.
E)None of these answers is the definition of E°.
A)the formal half-cell potential for compounds that are neither acids nor bases.
B)the formal half-cell potential at 20 C.
C)the formal half-cell potential at pH 7.
D)the inverse of E°.
E)None of these answers is the definition of E°.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
The half-cells below are connected via a salt-bridge and potentiometer.What is the voltage read by the potentiometer when AAg+ = 2.00 and AV2+ = 1.50? Ag+ + 1 e− → Ag (s)Eo = 0.7993 V
V2+ + 2 e− → V (s)Eo = −1.125 V
A)1)912 V
B)−0.326 V
C)−0.313 V
D)1)937 V
E)1)924 V
V2+ + 2 e− → V (s)Eo = −1.125 V
A)1)912 V
B)−0.326 V
C)−0.313 V
D)1)937 V
E)1)924 V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is NOT true for galvanic cells?
A)Galvanic cells are spontaneous.
B)Oxidation occurs at the anode and reduction occurs at the cathode.
C)Electrons move toward more negative electrical potential.
D)Galvanic cells are composed of two half-cells connected by salt bridge.
E)The salt bridge maintains electroneutrality throughout the cell.
A)Galvanic cells are spontaneous.
B)Oxidation occurs at the anode and reduction occurs at the cathode.
C)Electrons move toward more negative electrical potential.
D)Galvanic cells are composed of two half-cells connected by salt bridge.
E)The salt bridge maintains electroneutrality throughout the cell.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
A cell is constructed according to the cell diagram below.If the measured potential is 1.00 V,what is the pH of the dichromate cell? Fe|Fe2+ (aq,1M)|| 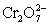 (aq 1 M),Cr2+ (aq,1 M),H+ (aq,x M)|Pt
(aq 1 M),Cr2+ (aq,1 M),H+ (aq,x M)|Pt 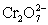 + 14 H+ + 6 e− ⇋ 2 Cr3+ + 7 H2O E° = 1.36 V
+ 14 H+ + 6 e− ⇋ 2 Cr3+ + 7 H2O E° = 1.36 V
Fe2+ + 2 e− ⇋ Fe (s)____________________ E° = −0.44 V
A)1)931
B)5)797
C)12.162
D)6)081
E)4)054
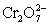 (aq 1 M),Cr2+ (aq,1 M),H+ (aq,x M)|Pt
(aq 1 M),Cr2+ (aq,1 M),H+ (aq,x M)|Pt 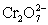 + 14 H+ + 6 e− ⇋ 2 Cr3+ + 7 H2O E° = 1.36 V
+ 14 H+ + 6 e− ⇋ 2 Cr3+ + 7 H2O E° = 1.36 VFe2+ + 2 e− ⇋ Fe (s)____________________ E° = −0.44 V
A)1)931
B)5)797
C)12.162
D)6)081
E)4)054

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
Which relationship is NOT correctly defined?
A)Work = E q
B)(G = -n · F · E)
C)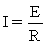
D)P = E I
E)q = n N F
A)Work = E q
B)(G = -n · F · E)
C)
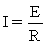
D)P = E I
E)q = n N F

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate the potential for the half-cell below for [ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://storage.examlex.com/TB4000/11ea6952_25ad_4b7b_a4b1_3547427d4325_TB4000_11.jpg) ] = 0.100 M,[
] = 0.100 M,[ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://storage.examlex.com/TB4000/11ea6952_25ad_4b7c_a4b1_11f3bec3a0eb_TB4000_11.jpg) ] = 0.500 M and the pH = 10.50.
] = 0.500 M and the pH = 10.50.
2![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://storage.examlex.com/TB4000/11ea6952_25ad_4b7d_a4b1_e907d93947e4_TB4000_11.jpg) + 3 H2O + 4 e− ⇋
+ 3 H2O + 4 e− ⇋ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://storage.examlex.com/TB4000/11ea6952_25ad_4b7e_a4b1_710927175ef4_TB4000_11.jpg) + 6 OH- E° = −0.566 V
+ 6 OH- E° = −0.566 V
![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://storage.examlex.com/TB4000/11ea6952_25ad_4b7b_a4b1_3547427d4325_TB4000_11.jpg) ] = 0.100 M,[
] = 0.100 M,[ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://storage.examlex.com/TB4000/11ea6952_25ad_4b7c_a4b1_11f3bec3a0eb_TB4000_11.jpg) ] = 0.500 M and the pH = 10.50.
] = 0.500 M and the pH = 10.50.2
![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://storage.examlex.com/TB4000/11ea6952_25ad_4b7d_a4b1_e907d93947e4_TB4000_11.jpg) + 3 H2O + 4 e− ⇋
+ 3 H2O + 4 e− ⇋ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://storage.examlex.com/TB4000/11ea6952_25ad_4b7e_a4b1_710927175ef4_TB4000_11.jpg) + 6 OH- E° = −0.566 V
+ 6 OH- E° = −0.566 V
Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
A galvanic cell with a large equilibrium constant has a____________________ cell potential.
A)negative
B)constant
C)zero voltage
D)positive
E)variable
A)negative
B)constant
C)zero voltage
D)positive
E)variable

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


