Exam 15: Fundamentals of Electrochemistry
Exam 1: The Analytical Process20 Questions
Exam 2: Chemical Measurements21 Questions
Exam 3: Tools of the Trade19 Questions
Exam 4: Experimental Error20 Questions
Exam 5: Statistics22 Questions
Exam 6: Quality Assurance and Calibration Methods20 Questions
Exam 7: Chemical Equilibrium20 Questions
Exam 8: Let the Titrations Begin20 Questions
Exam 9: Activity and the Systematic Treatment of Equilibrium20 Questions
Exam 10: Monoprotic Acid-Base Equilibria20 Questions
Exam 11: Polyprotic Acid-Base Equilibria20 Questions
Exam 12: Acid-Base Titrations20 Questions
Exam 13: Edta Titrations20 Questions
Exam 14: Advanced Topics in Equilibrium20 Questions
Exam 15: Fundamentals of Electrochemistry20 Questions
Exam 16: Electrodes and Potentiometry20 Questions
Exam 17: Redox Titrations20 Questions
Exam 18: Electroanalytical Techniques20 Questions
Exam 19: Fundamentals of Spectrophotometry23 Questions
Exam 20: Applications of Spectrophotometry20 Questions
Exam 21: Spectrophotometers20 Questions
Exam 22: Atomic Spectroscopy20 Questions
Exam 23: Mass Spectrometry20 Questions
Exam 24: Introduction to Analytical Separations20 Questions
Exam 25: Gas Chromatography20 Questions
Exam 26: High-Performance Liquid Chromatography20 Questions
Exam 27: Chromatographic Methods and Capillary Electrophoresis20 Questions
Exam 28: Gravimetric and Combustion Analysis20 Questions
Exam 29: Sample Preparation19 Questions
Select questions type
Which relationship is NOT correctly defined?
Free
(Multiple Choice)
4.7/5  (29)
(29)
Correct Answer:
B
For the cell diagram below write out and balance the reaction that occurs in the cell.
Pt|HOCl (aq,1 M),HClO2 (aq,1 M),H+ (aq,1 M)|| 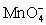 (aq,1 M),H+ (aq,1 M)| MnO2 (s)|Pt
(aq,1 M),H+ (aq,1 M)| MnO2 (s)|Pt
Free
(Essay)
4.9/5  (39)
(39)
Correct Answer:
2 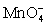 + 2 H+ + 3 HOCl ⇋ 2 MnO2 + 3 HClO2 + H2O
+ 2 H+ + 3 HOCl ⇋ 2 MnO2 + 3 HClO2 + H2O
Which is true when electrochemical cells are used as chemical probes?
I.All concentrations in the half-cells must be known except for the analyte of interest.
II. The analyte concentration is calculated from the equilibrium cell potential.
III. Equilibrium between the half-cells is established.
IV. Equilibrium within each half-cell is established and is assumed to remain at equilibrium.
V. Half-cells other than the S.H.E.can be used to determine pH.
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
B
All of the following are true for the measurement of standard reduction potentials,except:
I standard conditions for reduction potentials is 25 oC and A = 1 for all aqueous and gaseous species.
II reduction potentials are measured relative to the standard hydrogen electrode (S.H.E. ).
III standard reduction potentials are measured while connected to the negative electrode of the potentiometer.
IV the E° for the S.H.E.is defined as 0 volts.
(Multiple Choice)
4.8/5  (42)
(42)
For the line notation below,which is NOT true? Fe(s)| Fe2+ (aq,A = 1)|| Cu2+ (aq,A = 1)| Cu (s)
(Multiple Choice)
4.9/5  (43)
(43)
For the reaction below,the oxidizing agent is____________________ and it____________________ electrons,and the reducing agent is____________________ and it____________________ electrons. 8 H+ + 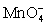 + 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2O
+ 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2O
(Multiple Choice)
4.7/5  (37)
(37)
Calculate E° for the half-cell below. O2 + 4 H+ + 4 e− ⇋ 2 H2O E° = 1.229 V
(Multiple Choice)
4.9/5  (44)
(44)
Calculate the standard potential when the two half-cells below are connected via a salt bridge and potentiometer.Which species are the reducing agent and oxidizing agent? 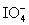 + 2 H+ + 2 e- ⇋
+ 2 H+ + 2 e- ⇋ 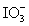 + H2O E° = 1.589 V
Mg(OH)2 (s)+ 2 e- ⇋ Mg (s)+ 2 OH- E° = -2.690 V
+ H2O E° = 1.589 V
Mg(OH)2 (s)+ 2 e- ⇋ Mg (s)+ 2 OH- E° = -2.690 V
(Essay)
4.9/5  (39)
(39)
A galvanic cell with a large equilibrium constant has a____________________ cell potential.
(Multiple Choice)
4.8/5  (34)
(34)
A cell is constructed according to the cell diagram below.If the measured potential is 1.00 V,what is the pH of the dichromate cell? Fe|Fe2+ (aq,1M)|| 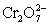 (aq 1 M),Cr2+ (aq,1 M),H+ (aq,x M)|Pt
(aq 1 M),Cr2+ (aq,1 M),H+ (aq,x M)|Pt 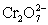 + 14 H+ + 6 e− ⇋ 2 Cr3+ + 7 H2O E° = 1.36 V
Fe2+ + 2 e− ⇋ Fe (s)____________________ E° = −0.44 V
+ 14 H+ + 6 e− ⇋ 2 Cr3+ + 7 H2O E° = 1.36 V
Fe2+ + 2 e− ⇋ Fe (s)____________________ E° = −0.44 V
(Multiple Choice)
4.7/5  (38)
(38)
Which is NOT correct for when the silver and vanadium half-cells are connected via a salt bridge and a potentiometer?
Ag+ + 1 e- → Ag (s)E° = 0.7993 V
V2+ + 2 e- → V (s)E° = −1.125 V
I Ag+ is reduced.
II V is oxidized.
III Eocell = 1.924 V
IV V2+ is reduced.
V Ag is oxidized.
(Multiple Choice)
4.7/5  (36)
(36)
Calculate the equilibrium constant for the reaction between Sn metal and Zn2+ solution. Sn + Zn2+ ⇋ Sn2+ + Zn
Sn2+ + 2 e− → Sn (s)E° = −0.141 V
Zn2+ + 2 e− → Zn (s)E° = −0.762 V
(Multiple Choice)
4.8/5  (37)
(37)
A bromide probe is constructed from a saturated silver bromide half-cell and a Ni2+/Ni half-cell.If the measured potential is 0.952 V and [Ni2+] = 1 M,what is the bromide concentration?
AgBr (s)+ e− ⇋ Ag(s)+ Br− E° = 0.071 V
Ni2+ + 2 e− ⇋ Ni (s)E° = −0.714 V
(Short Answer)
4.7/5  (44)
(44)
Calculate the potential for the half-cell below for [ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://storage.examlex.com/TB4000/11ea6952_25ad_4b7b_a4b1_3547427d4325_TB4000_11.jpg) ] = 0.100 M,[
] = 0.100 M,[ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://storage.examlex.com/TB4000/11ea6952_25ad_4b7c_a4b1_11f3bec3a0eb_TB4000_11.jpg) ] = 0.500 M and the pH = 10.50.
2
] = 0.500 M and the pH = 10.50.
2 ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://storage.examlex.com/TB4000/11ea6952_25ad_4b7d_a4b1_e907d93947e4_TB4000_11.jpg) + 3 H2O + 4 e− ⇋
+ 3 H2O + 4 e− ⇋ ![Calculate the potential for the half-cell below for [ ] = 0.100 M,[ ] = 0.500 M and the pH = 10.50. 2 + 3 H<sub>2</sub>O + 4 e<sup>−</sup> ⇋ + 6 OH<sup>-</sup> E° = −0.566 V](https://storage.examlex.com/TB4000/11ea6952_25ad_4b7e_a4b1_710927175ef4_TB4000_11.jpg) + 6 OH- E° = −0.566 V
+ 6 OH- E° = −0.566 V
(Short Answer)
4.8/5  (33)
(33)
Calculate Eo and K for the cell composed of a bromate/bromide half-cell and a chlorate/chlorine dioxide half-cell. 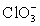 + 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V
+ 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V 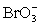 + 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
+ 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
(Short Answer)
4.8/5  (36)
(36)
The half-cells below are connected via a salt-bridge and potentiometer.What is the voltage read by the potentiometer when AAg+ = 2.00 and AV2+ = 1.50? Ag+ + 1 e− → Ag (s)Eo = 0.7993 V
V2+ + 2 e− → V (s)Eo = −1.125 V
(Multiple Choice)
4.7/5  (43)
(43)
For the silver half-reaction,Ag+ + 1 e- → Ag (s),when the concentration of silver cation is increased,the reduction potential:
(Multiple Choice)
4.8/5  (50)
(50)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)