Deck 18: The Laws of Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/90
Play
Full screen (f)
Deck 18: The Laws of Thermodynamics
1
When a gas expands adiabatically,
A)the internal (thermal)energy of the gas decreases.
B)the internal (thermal)energy of the gas increases.
C)it does no work.
D)work is done on the gas.
E)the temperature of the gas remains constant.
A)the internal (thermal)energy of the gas decreases.
B)the internal (thermal)energy of the gas increases.
C)it does no work.
D)work is done on the gas.
E)the temperature of the gas remains constant.
the internal (thermal)energy of the gas decreases.
2
A certain gas is compressed adiabatically.The amount of work done on the gas is 800 J.What is the change in the internal (thermal)energy of the gas?
A)800 J
B)-800 J
C)400 J
D)0 J
E)More information is needed to answer this question.
A)800 J
B)-800 J
C)400 J
D)0 J
E)More information is needed to answer this question.
800 J
3
The process shown on the pV diagram in the figure is an 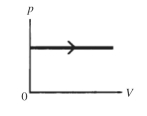
A)adiabatic expansion.
B)isothermal expansion.
C)isometric expansion.
D)isobaric expansion.

A)adiabatic expansion.
B)isothermal expansion.
C)isometric expansion.
D)isobaric expansion.
isobaric expansion.
4
A certain ideal gas has a molar specific heat at constant volume 7R/2.What is its molar specific heat at constant pressure?
A)5R/2
B)3R/2
C)8R
D)9R/2
E)6R
A)5R/2
B)3R/2
C)8R
D)9R/2
E)6R

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
5
The second law of thermodynamics leads us to conclude that
A)the total energy of the universe is constant.
B)disorder in the universe is increasing with the passage of time.
C)it is theoretically possible to convert heat into work with 100% efficiency.
D)the average temperature of the universe is increasing with the passage of time.
E)the entropy of the universe remains constant.
A)the total energy of the universe is constant.
B)disorder in the universe is increasing with the passage of time.
C)it is theoretically possible to convert heat into work with 100% efficiency.
D)the average temperature of the universe is increasing with the passage of time.
E)the entropy of the universe remains constant.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
6
In a given reversible process,the temperature of an ideal gas is kept constant as the gas is compressed to a smaller volume.Which one of the following statements about the gas is correct?
A)The gas must absorb heat from its surroundings.
B)The gas must release heat to its surroundings.
C)The pressure of the gas also stays constant.
D)The process is adiabatic.
E)It is impossible to predict on the basis of this data.
A)The gas must absorb heat from its surroundings.
B)The gas must release heat to its surroundings.
C)The pressure of the gas also stays constant.
D)The process is adiabatic.
E)It is impossible to predict on the basis of this data.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of the following is a true statement?
A)The second law of thermodynamics is a consequence of the first law of thermodynamics.
B)It is possible for heat to flow spontaneously from a hot body to a cold one or from a cold one to a hot one,depending on whether or not the process is reversible or irreversible.
C)It is not possible to convert work entirely into heat.
D)It is impossible to transfer heat from a cooler to a hotter body.
E)All of these statements are false.
A)The second law of thermodynamics is a consequence of the first law of thermodynamics.
B)It is possible for heat to flow spontaneously from a hot body to a cold one or from a cold one to a hot one,depending on whether or not the process is reversible or irreversible.
C)It is not possible to convert work entirely into heat.
D)It is impossible to transfer heat from a cooler to a hotter body.
E)All of these statements are false.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is a false statement?
A)Entropy is a quantitative measure of disorder.
B)The total entropy change in one cycle of a Carnot engine is zero.
C)The entropy of an isolated system must be conserved (it remains constant).
D)Entropy can be measured in units of J/K.
A)Entropy is a quantitative measure of disorder.
B)The total entropy change in one cycle of a Carnot engine is zero.
C)The entropy of an isolated system must be conserved (it remains constant).
D)Entropy can be measured in units of J/K.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
9
A certain ideal gas has a molar specific heat at constant pressure of 33.2 J/mol ∙ K.Its molar specific heat at constant volume is closest to which of the following values? (R = 8.31J/mol ∙ K)
A)41.9 J/mol ∙ K
B)16.6 J/mol ∙ K
C)25.1 J/mol ∙ K
D)24.9 J/mol ∙ K
E)49.8 J/mol ∙ K
A)41.9 J/mol ∙ K
B)16.6 J/mol ∙ K
C)25.1 J/mol ∙ K
D)24.9 J/mol ∙ K
E)49.8 J/mol ∙ K

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
10
For an ideal gas,
A)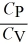 = 1 for all ideal gases.
= 1 for all ideal gases.
B) < 1 for all monatomic and diatomic gases.
< 1 for all monatomic and diatomic gases.
C)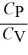 > 1 for all monatomic and diatomic gases.
> 1 for all monatomic and diatomic gases.
D)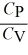 < 1 only for a monatomic gas.
< 1 only for a monatomic gas.
E)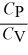 < 1 only for a diatomic gas.
< 1 only for a diatomic gas.
A)
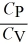 = 1 for all ideal gases.
= 1 for all ideal gases.B)
 < 1 for all monatomic and diatomic gases.
< 1 for all monatomic and diatomic gases.C)
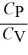 > 1 for all monatomic and diatomic gases.
> 1 for all monatomic and diatomic gases.D)
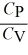 < 1 only for a monatomic gas.
< 1 only for a monatomic gas.E)
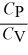 < 1 only for a diatomic gas.
< 1 only for a diatomic gas.
Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
11
A certain ideal gas has a molar specific heat at constant pressure of 7R/2.What is its molar specific heat at constant volume?
A)5R/2
B)3R/2
C)8R
D)9R/2
E)6R
A)5R/2
B)3R/2
C)8R
D)9R/2
E)6R

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
An ideal gas undergoes an isothermal expansion.During this process,its entropy
A)decreases.
B)remains unchanged.
C)increases.
D)cannot be predicted from the data given.
A)decreases.
B)remains unchanged.
C)increases.
D)cannot be predicted from the data given.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
13
An ideal gas is compressed isobarically to one-third of its initial volume.The resulting pressure will be
A)three times as large as the initial value.
B)equal to the initial value.
C)more than three times as large as the initial value.
D)impossible to predict on the basis of this data.
A)three times as large as the initial value.
B)equal to the initial value.
C)more than three times as large as the initial value.
D)impossible to predict on the basis of this data.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
14
For an ideal gas,
A)CP = CV for all ideal gases.
B)CP > CV for all ideal gases.
C)CP < CV for all ideal gases.
D)it depends on whether the gas is monatomic or diatomic.
A)CP = CV for all ideal gases.
B)CP > CV for all ideal gases.
C)CP < CV for all ideal gases.
D)it depends on whether the gas is monatomic or diatomic.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
15
The process shown on the TV graph in the figure is an 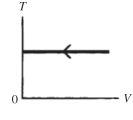
A)adiabatic compression.
B)isothermal compression.
C)isochoric compression.
D)isobaric compression.

A)adiabatic compression.
B)isothermal compression.
C)isochoric compression.
D)isobaric compression.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
16
A 10-L flask and a 1-L flask each contain two moles of ideal diatomic gas (but not the same gas)at 25°C.Which of the following statements about these gases must be true? (There could be more than one correct choice. )
A)The internal (thermal)energy of the gas in both flasks is the same.
B)The internal (thermal)energy of the gas in the larger flask is greater than the internal (thermal)energy of the gas in the smaller flask.
C)The internal (thermal)energy of the gas in the smaller flask is greater than the internal (thermal)energy of the gas in the larger flask.
D)The molecules in the larger flask have the same root-mean-square speed as those in the smaller flask.
E)The molecules in the smaller flask have the same average kinetic energy per molecule as those in the larger flask.
A)The internal (thermal)energy of the gas in both flasks is the same.
B)The internal (thermal)energy of the gas in the larger flask is greater than the internal (thermal)energy of the gas in the smaller flask.
C)The internal (thermal)energy of the gas in the smaller flask is greater than the internal (thermal)energy of the gas in the larger flask.
D)The molecules in the larger flask have the same root-mean-square speed as those in the smaller flask.
E)The molecules in the smaller flask have the same average kinetic energy per molecule as those in the larger flask.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
17
When water at 0°C freezes,the entropy of the water
A)increases.
B)decreases.
C)remains constant.
D)could either increase or decrease;it depends on other factors.
A)increases.
B)decreases.
C)remains constant.
D)could either increase or decrease;it depends on other factors.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
18
An ideal gas is compressed isothermally to one-third of its initial volume.The resulting pressure will be
A)three times as large as the initial value.
B)less than three times as large as the initial value.
C)more than three times as large as the initial value.
D)equal to the initial value.
E)impossible to predict on the basis of this data.
A)three times as large as the initial value.
B)less than three times as large as the initial value.
C)more than three times as large as the initial value.
D)equal to the initial value.
E)impossible to predict on the basis of this data.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
19
If the efficiency of a Carnot engine were to be 100%,the heat sink would have to be
A)at absolute zero.
B)at 0°C.
C)at 100°C.
D)infinitely hot.
A)at absolute zero.
B)at 0°C.
C)at 100°C.
D)infinitely hot.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
20
An important feature of the Carnot cycle is that
A)its efficiency can be 100%.
B)its efficiency depends only on the absolute temperature of the hot reservoir used.
C)its efficiency is determined by the temperatures of the hot and cold reservoirs between which it works and by the properties of the working substance used,and on nothing else.
D)it is an example of an irreversible process that can be analyzed exactly without approximations.
E)no engine can be more efficient than a Carnot engine operating between the same two temperatures.
A)its efficiency can be 100%.
B)its efficiency depends only on the absolute temperature of the hot reservoir used.
C)its efficiency is determined by the temperatures of the hot and cold reservoirs between which it works and by the properties of the working substance used,and on nothing else.
D)it is an example of an irreversible process that can be analyzed exactly without approximations.
E)no engine can be more efficient than a Carnot engine operating between the same two temperatures.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
21
In an isochoric process,the internal (thermal)energy of a gas decreases by 50 J.How much work is done by the gas during this process?
A)0 J
B)50 J
C)-50 J
D)25 J
E)-25 J
A)0 J
B)50 J
C)-50 J
D)25 J
E)-25 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
22
The gas in a perfectly insulated but flexible container does work at a rate of 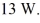
At what rate is the internal (thermal)energy of the gas changing?
A)-13 W
B)13 W
C)0 W
D)6.5 W
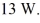
At what rate is the internal (thermal)energy of the gas changing?
A)-13 W
B)13 W
C)0 W
D)6.5 W

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
23
An external heat source supplies heat to a system at a rate of 187 W as the system does work at a rate of 131 W.At what rate is the internal (thermal)energy of the system changing?
A)-56 W
B)320 W
C)56 W
D)190 W
E)-320 W
A)-56 W
B)320 W
C)56 W
D)190 W
E)-320 W

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
24
The process shown on the pV diagram in the figure is 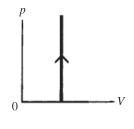
A)adiabatic.
B)isothermal.
C)isochoric.
D)isobaric.

A)adiabatic.
B)isothermal.
C)isochoric.
D)isobaric.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
25
In an adiabatic compression,200 J of work is done on a gas.What is the change in internal (thermal)energy of the gas during this compression?
A)0 J
B)100 J
C)200 J
D)-200 J
A)0 J
B)100 J
C)200 J
D)-200 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
26
The work done on an ideal gas system in an isothermal process is -400 J.What is the change in internal (thermal)energy of the gas?
A)0 J
B)-400 J
C)400 J
D)200 J
A)0 J
B)-400 J
C)400 J
D)200 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
27
During an isothermal process,5.0 J of heat is removed from an ideal gas.What is the change in internal (thermal)energy of the gas?
A)0 J
B)2.5 J
C)5.0 J
D)10 J
A)0 J
B)2.5 J
C)5.0 J
D)10 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
28
A fluid in an insulated,flexible bottle is heated by a high resistance wire and expands.If 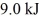
Of heat is applied to the system and it does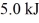
Of work,how much does the internal (thermal)energy of the fluid change?
A)4.0 kJ
B)14 kJ
C)-4.0 kJ
D)45 kJ
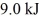
Of heat is applied to the system and it does
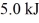
Of work,how much does the internal (thermal)energy of the fluid change?
A)4.0 kJ
B)14 kJ
C)-4.0 kJ
D)45 kJ

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
29
During an isothermal process,5.0 J of heat is removed from an ideal gas.What is the work done by the gas in the process?
A)0 J
B)5.0 J
C)-5.0 J
D)-10 J
A)0 J
B)5.0 J
C)-5.0 J
D)-10 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
30
A gas is taken through the cycle shown in the pV diagram in the figure.During one cycle,how much work is done by the gas? 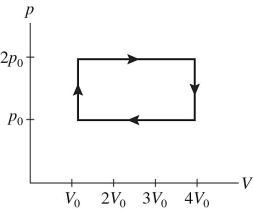
A)p0V0
B)2 p0V0
C)3 p0V0
D)4 p0V0

A)p0V0
B)2 p0V0
C)3 p0V0
D)4 p0V0

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
31
A nuclear power plant has an actual efficiency of 33%.If 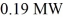
Of energy are released from fission,how much electric power does the power plant produce?
A)0.063 MW
B)6.3 MW
C)25 MW
D)0.25 MW
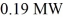
Of energy are released from fission,how much electric power does the power plant produce?
A)0.063 MW
B)6.3 MW
C)25 MW
D)0.25 MW

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
32
A certain heat engine extracts 1.30 kJ of heat from a hot temperature reservoir and discharges 0.70 kJ of heat to a cold temperature reservoir.What is the efficiency of this engine?
A)46%
B)54%
C)86%
D)27%
E)13%
A)46%
B)54%
C)86%
D)27%
E)13%

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
33
A heat engine with an efficiency of 30% performs 2500 J of work.How much heat is discharged to the lower temperature reservoir?
A)5800 J
B)8300 J
C)750 J
D)1400 J
E)7100 J
A)5800 J
B)8300 J
C)750 J
D)1400 J
E)7100 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
34
A heat engine receives 7000 J of heat and loses 3000 J in each cycle.What is the efficiency of this engine?
A)57%
B)30%
C)70%
D)43%
A)57%
B)30%
C)70%
D)43%

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
35
An expansion process on an ideal diatomic ideal gas for which CV = 5/2 R has a linear path between the initial and final coordinates on a pV diagram.The coordinates of the initial state are: the pressure is 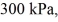
The volume is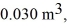
And the temperature is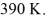
The final pressure is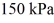
And the final temperature is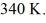
What is the change in the internal (thermal)energy of the gas,during this process? (R = 8.31 J/mol ∙ K)
A)-2,900 J
B)-1,700 J
C)2,900 J
D)1,700 J
E)0 J
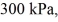
The volume is
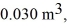
And the temperature is
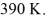
The final pressure is
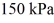
And the final temperature is
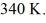
What is the change in the internal (thermal)energy of the gas,during this process? (R = 8.31 J/mol ∙ K)
A)-2,900 J
B)-1,700 J
C)2,900 J
D)1,700 J
E)0 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
36
During an isochoric process,the internal (thermal)energy of a gas decreases by 50 J.How much heat is added to the gas during this process?
A)0 J
B)50 J
C)-50 J
D)25 J
E)-25 J
A)0 J
B)50 J
C)-50 J
D)25 J
E)-25 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
37
An athlete doing push-ups performs 650 kJ of work and loses 425 kJ of heat.What is the change in the internal (thermal)energy of the athlete?
A)-225 kJ
B)-1075 kJ
C)1075 kJ
D)225 kJ
E)276 kJ
A)-225 kJ
B)-1075 kJ
C)1075 kJ
D)225 kJ
E)276 kJ

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
38
An ideal gas undergoes an adiabatic process while doing 25 J of work.What is the change in the internal (thermal)energy of the gas?
A)0 J
B)25 J
C)-25 J
D)50 J
E)-50 J
A)0 J
B)25 J
C)-25 J
D)50 J
E)-50 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
39
A fixed amount of an ideal monatomic gas is maintained at constant volume as it is cooled by 50 K.This feat is accomplished by removing 400 J of energy from the gas.How much work is done by the gas during this process?
A)0 J
B)400 J
C)-400 J
D)-200 J
E)200 J
A)0 J
B)400 J
C)-400 J
D)-200 J
E)200 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
40
Two processes are shown on the pV diagram in the figure.One of them is an adiabat and the other one is an isotherm.Which process is the isotherm? 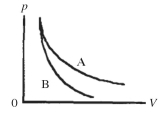
A)process A
B)process B
C)The processes shown are neither isotherms nor adiabats.
D)It is not possible to tell without knowing if the gas is monatomic or diatomic.

A)process A
B)process B
C)The processes shown are neither isotherms nor adiabats.
D)It is not possible to tell without knowing if the gas is monatomic or diatomic.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
41
A refrigerator has a coefficient of performance equal to 4.2.How much work must be done on the operating gas in the refrigerator in order to remove 250 J of heat from the interior compartment?
A)60 J
B)120 J
C)250 J
D)480 J
E)1050 J
A)60 J
B)120 J
C)250 J
D)480 J
E)1050 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
42
What is the efficiency of an ideal Carnot engine operating between a reservoir in which ice and water coexist,and a reservoir in which water and steam coexist? The pressure is constant at 1.0 atm for both reservoirs.
A)27%
B)0.27%
C)100%
D)1.0%
E)15%
A)27%
B)0.27%
C)100%
D)1.0%
E)15%

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
43
An air conditioner with a coefficient of performance of 3.50 uses 30.0 kW of power to operate.What power is it discharging to the outdoors?
A)30.0 kW
B)75.0 kW
C)105 kW
D)135 kW
E)210 kW
A)30.0 kW
B)75.0 kW
C)105 kW
D)135 kW
E)210 kW

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
44
An ideal Carnot engine operates between a high temperature reservoir at 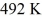
And a river with water at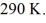
If it absorbs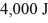
Of heat each cycle,how much work per cycle does it perform?
A)1,642 J
B)2,358 J
C)1,483 J
D)2,517 J
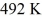
And a river with water at
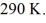
If it absorbs
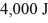
Of heat each cycle,how much work per cycle does it perform?
A)1,642 J
B)2,358 J
C)1,483 J
D)2,517 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
45
A heat engine having the maximum possible efficiency has an efficiency of 35.0% when operating between two heat reservoirs.If the temperature of the hot reservoir is 700 K,what is the temperature of the cold reservoir?
A)200 K
B)245 K
C)350 K
D)455 K
E)600 K
A)200 K
B)245 K
C)350 K
D)455 K
E)600 K

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
46
An ideal Carnot engine has an efficiency of 83.0% and performs 4500 J of work every cycle.How much energy is discharged to the lower temperature reservoir every cycle?
A)920 J
B)830 J
C)740 J
D)3700 J
E)5400 J
A)920 J
B)830 J
C)740 J
D)3700 J
E)5400 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
47
An ideal Carnot engine extracts 529 J of heat from a high-temperature reservoir during each cycle,and rejects 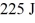
Of heat to a low-temperature reservoir during the same cycle.What is the efficiency of the engine?
A)0.57
B)1.35
C)2.35
D)0.7
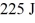
Of heat to a low-temperature reservoir during the same cycle.What is the efficiency of the engine?
A)0.57
B)1.35
C)2.35
D)0.7

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
48
During each cycle of operation,a refrigerator absorbs 230 J of heat from the freezer and expels 356 J of heat to the room.How much work input is required in each cycle?
A)712 J
B)586 J
C)460 J
D)126 J
A)712 J
B)586 J
C)460 J
D)126 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
49
Suppose that the Department of Energy develops a new reversible engine that has a coefficient of performance (COP)of 4.0 when operated as a refrigerator and a COP of 5.0 when operated as a heat pump.What is its thermal efficiency when operated as a heat engine doing work?
A)10%
B)20%
C)25%
D)45%
E)80%
A)10%
B)20%
C)25%
D)45%
E)80%

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
50
An ideal Carnot heat engine has an efficiency of 0.600.If it operates between a deep lake with a constant temperature of 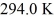
And a hot reservoir,what is the temperature of the hot reservoir?
A)735 K
B)490 K
C)470 K
D)784 K
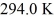
And a hot reservoir,what is the temperature of the hot reservoir?
A)735 K
B)490 K
C)470 K
D)784 K

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
51
For a certain ideal Carnot engine,the hot reservoir is 35°C higher than the cold reservoir.If this engine is to have an efficiency of 20%,what must be the temperature of the hot reservoir?
A)70.0 K
B)140 K
C)175 K
D)210 K
E)245 K
A)70.0 K
B)140 K
C)175 K
D)210 K
E)245 K

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
52
An ideal Carnot engine operates between a warm reservoir at 233 K and a colder reservoir.During each cycle,this engine extracts 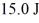
Of heat from the warm reservoir and does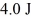
Of work.What is the temperature of the colder reservoir?
A)171 K
B)62 K
C)47 K
D)67 K
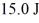
Of heat from the warm reservoir and does
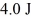
Of work.What is the temperature of the colder reservoir?
A)171 K
B)62 K
C)47 K
D)67 K

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
53
An ideal Carnot engine operating between a warm reservoir of unknown temperature and a cold reservoir at 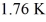
Has an efficiency of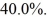
What is the temperature of the warm reservoir?
A)2.93 K
B)0.0500 K
C)106 K
D)0.0400 K
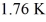
Has an efficiency of
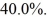
What is the temperature of the warm reservoir?
A)2.93 K
B)0.0500 K
C)106 K
D)0.0400 K

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
54
A Carnot engine operates between two reservoirs with unknown temperatures.If the Carnot engine operates at 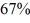
Efficiency,what is the ratio of the absolute temperatures of the reservoirs,Tc/Th?
A)0.33
B)0.0012
C)0.0025
D)0.67
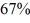
Efficiency,what is the ratio of the absolute temperatures of the reservoirs,Tc/Th?
A)0.33
B)0.0012
C)0.0025
D)0.67

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
55
An ideal Carnot engine operating between a warm reservoir of unknown temperature and a cold reservoir at 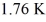
Has an efficiency of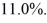
What is the temperature of the warm reservoir?
A)1.98 K
B)0.180 K
C)157 K
D)0.160 K
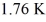
Has an efficiency of
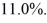
What is the temperature of the warm reservoir?
A)1.98 K
B)0.180 K
C)157 K
D)0.160 K

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
56
A heat engine having the maximum possible efficiency has an efficiency of 25% when operating between two heat reservoirs.If the temperature of the cold reservoir is 300 K,what is the temperature of the hot reservoir?
A)350 K
B)375 K
C)400 K
D)450 K
E)500 K
A)350 K
B)375 K
C)400 K
D)450 K
E)500 K

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
57
Two ideal Carnot heat engines have the same efficiency.One operates between 5.0 × 102 K and 3.0 × 102 K,and the other between 4.0 × 102 K and some lower temperature.What is the lower temperature?
A)200 K
B)220 K
C)240 K
D)260 K
E)280 K
A)200 K
B)220 K
C)240 K
D)260 K
E)280 K

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
58
An ideal Carnot heat engine operates between 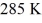
And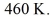
What is its efficiency?
A)0.38
B)0.62
C)0.61
D)1.61
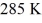
And
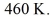
What is its efficiency?
A)0.38
B)0.62
C)0.61
D)1.61

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
59
The ocean thermal energy conversion project uses the surface water near tropical islands with a temperature of 20°C as the hot temperature reservoir,and the water at some depth,with a temperature of 5.0°C,as the cold temperature reservoir for a heat engine.What is the maximum possible efficiency of an engine running between those two temperatures?
A)4.7%
B)5.1%
C)7.9%
D)15%
E)30%
A)4.7%
B)5.1%
C)7.9%
D)15%
E)30%

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
60
The "hot shot" heat engine operating between 40°C and 380°C has an efficiency that is 60% of that of an ideal Carnot engine operating between the same temperatures.If the "hot shot" engine absorbs heat at a rate of 60 kW,at what rate does it exhaust heat?
A)36 kW
B)41 kW
C)57 kW
D)60 kW
A)36 kW
B)41 kW
C)57 kW
D)60 kW

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
61
A sealed 87- 
Tank is filled with 6,000 moles of ideal oxygen gas O2 at an initial temperature of 270 K.The gas is heated to a final temperature of 460 K.The atomic mass of oxygen is 16.0 g/mol.How much heat is transferred to the gas during this process? (R = 8.31 J/mol ∙ K)
A)24 MJ
B)28 MJ
C)19 MJ
D)14 MJ
E)9.1 MJ
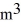
Tank is filled with 6,000 moles of ideal oxygen gas O2 at an initial temperature of 270 K.The gas is heated to a final temperature of 460 K.The atomic mass of oxygen is 16.0 g/mol.How much heat is transferred to the gas during this process? (R = 8.31 J/mol ∙ K)
A)24 MJ
B)28 MJ
C)19 MJ
D)14 MJ
E)9.1 MJ

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
62
A container with rigid walls is filled with 4.00 mol of air at 17°C with CV = 2.5R.What is the final temperature of the air if its internal energy is increased by 28 kJ? (R = 8.31 J/mol ∙ K)
A)337°C
B)354°C
C)337 K
D)354 K
E)610 K
A)337°C
B)354°C
C)337 K
D)354 K
E)610 K

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
63
A Carnot air conditioner has a coefficient of performance of 17.0 and removes 72.0 MJ of heat from the interior of a house every hour.How much power does it consume?
A)1180 W
B)1320 W
C)520 kW
D)3.14 MW
E)1.25 MW
A)1180 W
B)1320 W
C)520 kW
D)3.14 MW
E)1.25 MW

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
64
How much heat is required to increase the temperature of 1.70 moles of an ideal monatomic gas by 23.0 K at constant pressure? (R = 8.31 J/mol ∙ K)
A)812 J
B)346 J
C)751 J
D)391 J
E)290 J
A)812 J
B)346 J
C)751 J
D)391 J
E)290 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
65
An ideal reversible heat pump is taking heat from the outside air at -10.0°C and discharging it into the house at 18.0°C.What is the coefficient of performance of this heat pump?
A)10.4
B)9.44
C)0.644
D)0.533
E)0.0962
A)10.4
B)9.44
C)0.644
D)0.533
E)0.0962

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
66
A rigid container is filled with 4.0 mol of air with CV = 2.5R.How much does the internal (thermal)energy of the air change if its temperature rises from 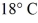
To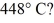
(R = 8.31 J/mol ∙ K)
A)35,800 J
B)430 J
C)3,580 J
D)8,940 J
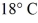
To
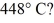
(R = 8.31 J/mol ∙ K)
A)35,800 J
B)430 J
C)3,580 J
D)8,940 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
67
If we add 700 J of heat to 12 moles of an ideal monatomic gas at constant volume,what will be the change in temperature of the gas? (R = 8.31 J/mol ∙ K)
A)4.7 K
B)5.2 K
C)5.8 J
D)6.8 K
E)9.3 K
A)4.7 K
B)5.2 K
C)5.8 J
D)6.8 K
E)9.3 K

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
68
During each cycle,the compressor in a certain ideal Carnot refrigerator performs 480 J of work to remove 150 J of heat from the interior of the refrigerator.How much heat do the coils behind the refrigerator discharge into the kitchen each cycle?
A)110 J
B)150 J
C)330 J
D)480 J
E)630 J
A)110 J
B)150 J
C)330 J
D)480 J
E)630 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
69
A heat pump with a performance coefficient (COP)of 4.9 absorbs heat from the atmosphere at a rate of 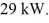
At what rate is work being done to run this heat pump?
A)6 kW
B)142 kW
C)113 kW
D)35 kW
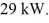
At what rate is work being done to run this heat pump?
A)6 kW
B)142 kW
C)113 kW
D)35 kW

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
70
An ideal Carnot air conditioner operates between an indoor temperature of 20°C and an outdoor temperature of 39°C.How much energy does it use to remove 2000 J of heat from the interior of the house?
A)105 J
B)130 J
C)780 J
D)520 J
E)340 J
A)105 J
B)130 J
C)780 J
D)520 J
E)340 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
71
The figure shows a pV diagram for 2.9 g of ideal oxygen gas O2 in a sealed container.The temperature of state 1 is 76° C,the atomic mass of the oxygen atom is 16 g/mol,and R = 8.31 J/mol ∙ K.What are the temperatures T3 and T4? 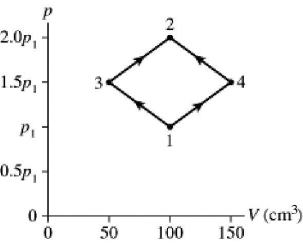
A)-11° C and 510° C
B)57° C and 170° C
C)260° C and 790° C
D)38° C and 110° C

A)-11° C and 510° C
B)57° C and 170° C
C)260° C and 790° C
D)38° C and 110° C

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
72
An ideal Carnot engine is operated as an air conditioner to cool a house in the summer.The air conditioner removes 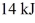
Of heat per second from the house,and maintains the inside temperature at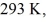
While the outside temperature is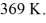
The power required to run the air conditioner under these operating conditions is closest to
A)3,600 W.
B)4,400 W.
C)5,100 W.
D)5,800 W.
E)6,600 W.
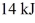
Of heat per second from the house,and maintains the inside temperature at
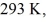
While the outside temperature is
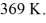
The power required to run the air conditioner under these operating conditions is closest to
A)3,600 W.
B)4,400 W.
C)5,100 W.
D)5,800 W.
E)6,600 W.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
73
An ideal Carnot refrigerator with a performance coefficient (COP)of 5.0 cools items inside of it to 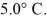
What is the high temperature needed to operate this refrigerator?
A)61° C
B)1,395° C
C)6° C
D)30° C
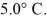
What is the high temperature needed to operate this refrigerator?
A)61° C
B)1,395° C
C)6° C
D)30° C

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
74
An ideal Carnot engine is operated as a heat pump to heat a room in the winter.The heat pump delivers heat to the room at the rate of 47 kJ per second and maintains the room at a temperature of 293 K when the outside temperature is 237 K.The power requirement to run the heat pump under these operating conditions is closest to
A)9000 W.
B)7100 W.
C)20,000 W.
D)15,000 W.
E)11,000 W.
A)9000 W.
B)7100 W.
C)20,000 W.
D)15,000 W.
E)11,000 W.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
75
The temperature of an ideal gas in a sealed rigid 0.60- 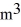
Container is reduced from 460 K to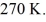
The final pressure of the gas is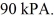
The molar heat capacity at constant volume of the gas is 28.0 J/mol ∙ K.How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)
A)-130 kJ
B)-170 kJ
C)130 kJ
D)170 kJ
E)0 kJ
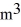
Container is reduced from 460 K to
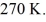
The final pressure of the gas is
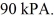
The molar heat capacity at constant volume of the gas is 28.0 J/mol ∙ K.How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)
A)-130 kJ
B)-170 kJ
C)130 kJ
D)170 kJ
E)0 kJ

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
76
The figure shows a pV diagram for 8.3 g of ideal nitrogen gas N2 in a sealed container.The temperature of state 1 is 59°C,the atomic mass of the nitrogen atom is 14 g/mol,and R = 8.31 J/mol ∙ K.What are (a)pressure p1 and (b)temperature T2? 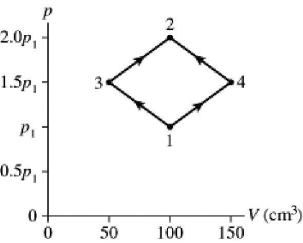
A)(a)81 atm,(b)660°C
B)(a)14 atm,(b)660°C
C)(a)81 atm,(b)120°C
D)(a)14 atm,(b)120°C

A)(a)81 atm,(b)660°C
B)(a)14 atm,(b)660°C
C)(a)81 atm,(b)120°C
D)(a)14 atm,(b)120°C

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
77
How much heat is required to raise the temperature of 2.00 moles of an ideal monatomic gas by 10°C at constant volume? (R = 8.31 J/mol ∙ K).
A)249 J
B)416 J
C)208 J
D)200 J
E)125 J
A)249 J
B)416 J
C)208 J
D)200 J
E)125 J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
78
A heat pump absorbs heat from the atmosphere at a rate of 30 kW.If work is being done to run this heat pump at a rate of 7.7 kW,what is the coefficient of performance (COP)of the heat pump?
A)3.9
B)4.9
C)2.9
D)0.26
E)22
A)3.9
B)4.9
C)2.9
D)0.26
E)22

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
79
A sample of ideal monatomic gas is cooled by 50.0°C at constant volume by removing 831 J of energy from it.How many moles of gas are in the sample? (R = 8.31 J/mol ∙ K)
A)2.50 mol
B)1.50 mol
C)1.33 mol
D)1.00 mol
A)2.50 mol
B)1.50 mol
C)1.33 mol
D)1.00 mol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
80
The figure shows a pV diagram for 2.6 g of ideal helium gas that undergoes the process 1 → 2 → 3.Find the value of volume V3.The atomic mass of helium is 4.0 g/mol,and R = 8.31 J/mol ∙ K. 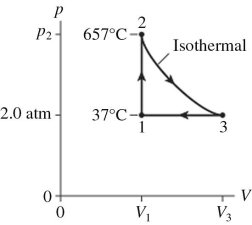
A)25 L
B)99 L
C)50 L
D)12 L

A)25 L
B)99 L
C)50 L
D)12 L

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck


