Exam 18: The Laws of Thermodynamics
Exam 1: Introduction to Physics98 Questions
Exam 2: One-Dimensional Kinematics138 Questions
Exam 3: Vectors in Physics69 Questions
Exam 4: Two-Dimensional Kinematics51 Questions
Exam 5: Newtons Laws of Motion54 Questions
Exam 6: Applications of Newtons Laws104 Questions
Exam 7: Work and Kinetic Energy55 Questions
Exam 8: Potential Energy and Conservation of Energy62 Questions
Exam 9: Linear Momentum and Collisions114 Questions
Exam 10: Rotational Kinematics and Energy60 Questions
Exam 11: Rotational Dynamics and Static Equilibrium69 Questions
Exam 12: Gravity53 Questions
Exam 13: Oscillations About Equilibrium79 Questions
Exam 14: Waves and Sound142 Questions
Exam 15: Fluids103 Questions
Exam 16: Temperature and Heat110 Questions
Exam 17: Phases and Phase Changes93 Questions
Exam 18: The Laws of Thermodynamics90 Questions
Exam 19: Electric Charges, Forces, and Fields75 Questions
Exam 20: Electric Potential and Electric124 Questions
Exam 21: Electric Current and Direct-Current Circuits228 Questions
Exam 22: Magnetism147 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction98 Questions
Exam 24: Alternating-Current Circuits72 Questions
Exam 25: Electromagnetic Waves63 Questions
Exam 26: Geometrical Optics133 Questions
Exam 27: Optical Instruments103 Questions
Exam 28: Physical Optics: Interference and Diffraction119 Questions
Exam 29: Relativity98 Questions
Exam 30: Quantum Physics88 Questions
Exam 31: Atomic Physics97 Questions
Exam 32: Nuclear Physics and Nuclear Radiation137 Questions
Select questions type
A certain ideal gas has a molar specific heat at constant volume 7R/2.What is its molar specific heat at constant pressure?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
D
What is the efficiency of an ideal Carnot engine operating between a reservoir in which ice and water coexist,and a reservoir in which water and steam coexist? The pressure is constant at 1.0 atm for both reservoirs.
Free
(Multiple Choice)
4.7/5  (31)
(31)
Correct Answer:
A
An ideal Carnot engine operating between a warm reservoir of unknown temperature and a cold reservoir at 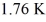 Has an efficiency of
Has an efficiency of 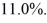 What is the temperature of the warm reservoir?
What is the temperature of the warm reservoir?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
A
An ideal Carnot air conditioner operates between an indoor temperature of 20°C and an outdoor temperature of 39°C.How much energy does it use to remove 2000 J of heat from the interior of the house?
(Multiple Choice)
4.7/5  (38)
(38)
During an isothermal process,5.0 J of heat is removed from an ideal gas.What is the work done by the gas in the process?
(Multiple Choice)
4.8/5  (40)
(40)
During each cycle of operation,a refrigerator absorbs 230 J of heat from the freezer and expels 356 J of heat to the room.How much work input is required in each cycle?
(Multiple Choice)
4.9/5  (27)
(27)
A heat engine having the maximum possible efficiency has an efficiency of 35.0% when operating between two heat reservoirs.If the temperature of the hot reservoir is 700 K,what is the temperature of the cold reservoir?
(Multiple Choice)
4.7/5  (32)
(32)
A rigid container is filled with 4.0 mol of air with CV = 2.5R.How much does the internal (thermal)energy of the air change if its temperature rises from 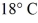 To
To 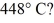 (R = 8.31 J/mol ∙ K)
(R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.9/5  (33)
(33)
A 10-L flask and a 1-L flask each contain two moles of ideal diatomic gas (but not the same gas)at 25°C.Which of the following statements about these gases must be true? (There could be more than one correct choice. )
(Multiple Choice)
5.0/5  (33)
(33)
During an isochoric process,the internal (thermal)energy of a gas decreases by 50 J.How much heat is added to the gas during this process?
(Multiple Choice)
4.9/5  (28)
(28)
An expansion process on an ideal diatomic ideal gas for which CV = 5/2 R has a linear path between the initial and final coordinates on a pV diagram.The coordinates of the initial state are: the pressure is 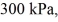 The volume is
The volume is 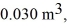 And the temperature is
And the temperature is 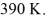 The final pressure is
The final pressure is 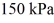 And the final temperature is
And the final temperature is 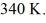 What is the change in the internal (thermal)energy of the gas,during this process? (R = 8.31 J/mol ∙ K)
What is the change in the internal (thermal)energy of the gas,during this process? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.9/5  (37)
(37)
The temperature of an ideal gas in a sealed rigid 0.60- 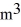 Container is reduced from 460 K to
Container is reduced from 460 K to 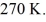 The final pressure of the gas is
The final pressure of the gas is 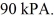 The molar heat capacity at constant volume of the gas is 28.0 J/mol ∙ K.How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)
The molar heat capacity at constant volume of the gas is 28.0 J/mol ∙ K.How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (35)
(35)
Two ideal Carnot heat engines have the same efficiency.One operates between 5.0 × 102 K and 3.0 × 102 K,and the other between 4.0 × 102 K and some lower temperature.What is the lower temperature?
(Multiple Choice)
4.8/5  (33)
(33)
A refrigerator has a coefficient of performance equal to 4.2.How much work must be done on the operating gas in the refrigerator in order to remove 250 J of heat from the interior compartment?
(Multiple Choice)
4.9/5  (40)
(40)
A container with rigid walls is filled with 4.00 mol of air at 17°C with CV = 2.5R.What is the final temperature of the air if its internal energy is increased by 28 kJ? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.9/5  (35)
(35)
In a given reversible process,the temperature of an ideal gas is kept constant as the gas is compressed to a smaller volume.Which one of the following statements about the gas is correct?
(Multiple Choice)
4.9/5  (37)
(37)
An athlete doing push-ups performs 650 kJ of work and loses 425 kJ of heat.What is the change in the internal (thermal)energy of the athlete?
(Multiple Choice)
4.9/5  (42)
(42)
A heat pump absorbs heat from the atmosphere at a rate of 30 kW.If work is being done to run this heat pump at a rate of 7.7 kW,what is the coefficient of performance (COP)of the heat pump?
(Multiple Choice)
4.9/5  (32)
(32)
A fixed amount of an ideal monatomic gas is maintained at constant volume as it is cooled by 50 K.This feat is accomplished by removing 400 J of energy from the gas.How much work is done by the gas during this process?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)