Deck 30: Quantum Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/88
Play
Full screen (f)
Deck 30: Quantum Physics
1
As the temperature of a blackbody increases,what happens to the peak wavelength of the light it radiates?
A)It gets longer.
B)It gets shorter.
C)The wavelength is not affected by the temperature of the object.
A)It gets longer.
B)It gets shorter.
C)The wavelength is not affected by the temperature of the object.
It gets shorter.
2
Which of the following actions will increase the energy of a photon? (There could be more than one correct choice. )
A)Increase its wavelength.
B)Increase its frequency.
C)Decrease its wavelength.
D)Decrease its frequency.
E)Increase its speed.
A)Increase its wavelength.
B)Increase its frequency.
C)Decrease its wavelength.
D)Decrease its frequency.
E)Increase its speed.
Increase its frequency.
Decrease its wavelength.
Decrease its wavelength.
3
A blue laser beam is incident on a metallic surface,causing electrons to be ejected from the metal.If the frequency of the laser beam is increased while the intensity of the beam is held fixed,
A)the rate of ejected electrons will decrease and their maximum kinetic energy will increase.
B)the rate of ejected electrons will remain the same but their maximum kinetic energy will increase.
C)the rate of ejected electrons will increase and their maximum kinetic energy will increase.
D)the rate of ejected electrons will remain the same but their maximum kinetic energy will decrease.
A)the rate of ejected electrons will decrease and their maximum kinetic energy will increase.
B)the rate of ejected electrons will remain the same but their maximum kinetic energy will increase.
C)the rate of ejected electrons will increase and their maximum kinetic energy will increase.
D)the rate of ejected electrons will remain the same but their maximum kinetic energy will decrease.
the rate of ejected electrons will decrease and their maximum kinetic energy will increase.
4
Two sources emit beams of light of wavelength 550 nm.The light from source A has an intensity of 10 µW/m2,and the light from source B has an intensity of 20 µW/m2.This is all we know about the two beams.Which of the following statements about these beams are correct? (There could be more than one correct choice. )
A)Beam B carries twice as many photons per second as beam A.
B)A photon in beam B has twice the energy of a photon in beam A.
C)The frequency of the light in beam B is twice as great as the frequency of the light in beam A.
D)A photon in beam B has the same energy as a photon in beam A.
E)None of the above statements are true.
A)Beam B carries twice as many photons per second as beam A.
B)A photon in beam B has twice the energy of a photon in beam A.
C)The frequency of the light in beam B is twice as great as the frequency of the light in beam A.
D)A photon in beam B has the same energy as a photon in beam A.
E)None of the above statements are true.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following actions will increase the de Broglie wavelength of a speck of dust? (There could be more than one correct choice. )
A)Increase its mass.
B)Increase its speed.
C)Decrease its mass.
D)Decrease its speed.
E)Decrease its momentum.
A)Increase its mass.
B)Increase its speed.
C)Decrease its mass.
D)Decrease its speed.
E)Decrease its momentum.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
6
When the surface of a metal is exposed to blue light,electrons are emitted.If the intensity of the blue light is increased,which of the following things will also increase?
A)the number of electrons ejected per second
B)the maximum kinetic energy of the ejected electrons
C)the time lag between the onset of the absorption of light and the ejection of electrons
D)the work function of the metal
E)all of the above
A)the number of electrons ejected per second
B)the maximum kinetic energy of the ejected electrons
C)the time lag between the onset of the absorption of light and the ejection of electrons
D)the work function of the metal
E)all of the above

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
7
Protons are being accelerated in a particle accelerator.When the energy of the protons is doubled,their de Broglie wavelength will
A)increase by a factor of 4.
B)increase by a factor of 2.
C)decrease by a factor of 2.
D)increase by a factor of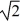 .
.
E)decrease by a factor of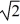 .
.
A)increase by a factor of 4.
B)increase by a factor of 2.
C)decrease by a factor of 2.
D)increase by a factor of
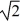 .
.E)decrease by a factor of
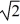 .
.
Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
8
If a proton and an electron have the same speed,which one has the longer de Broglie wavelength?
A)the electron
B)the proton
C)It is the same for both of them.
A)the electron
B)the proton
C)It is the same for both of them.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
9
A beam of light falling on a metal surface is causing electrons to be ejected from the surface.If we now double the frequency of the light,which of the following statements are correct? (There could be more than one correct choice. )
A)The kinetic energy of the ejected electrons doubles.
B)The speed of the ejected electrons doubles.
C)The number of electrons ejected per second doubles.
D)Twice as many photons hit the metal surface as before.
E)None of the above things occur.
A)The kinetic energy of the ejected electrons doubles.
B)The speed of the ejected electrons doubles.
C)The number of electrons ejected per second doubles.
D)Twice as many photons hit the metal surface as before.
E)None of the above things occur.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
10
If the wavelength of a photon is doubled,what happens to its energy?
A)It is reduced to one-half of its original value.
B)It stays the same.
C)It is doubled.
D)It is increased to four times its original value.
E)It is reduced to one-fourth of its original value.
A)It is reduced to one-half of its original value.
B)It stays the same.
C)It is doubled.
D)It is increased to four times its original value.
E)It is reduced to one-fourth of its original value.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
11
Monochromatic light falls on a metal surface and electrons are ejected.If the intensity of the light is increased,what will happen to the ejection rate and maximum energy of the electrons?
A)greater rate;same maximum energy
B)same rate;greater maximum energy
C)greater rate;greater maximum energy
D)same rate;same maximum energy
A)greater rate;same maximum energy
B)same rate;greater maximum energy
C)greater rate;greater maximum energy
D)same rate;same maximum energy

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
12
If the wavelength of a photon in vacuum is the same as the de Broglie wavelength of an electron,which one is traveling faster through space?
A)The electron because it has more mass.
B)The photon because photons always travel through space faster than electrons.
C)They both have the same speed.
A)The electron because it has more mass.
B)The photon because photons always travel through space faster than electrons.
C)They both have the same speed.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
13
Two identical metal bars are heated up until they are both glowing.One of them is "red hot" and the other is "blue hot." Which one is hotter,the one that glows red or the one that glows blue?
A)the red one
B)the blue one
C)We cannot tell without knowing more about the two bars.
A)the red one
B)the blue one
C)We cannot tell without knowing more about the two bars.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
14
If you double the frequency of the light in a laser beam,but keep the number of photons per second in the beam fixed,which of the following statements are correct? (There could be more than one correct choice. )
A)The power in the beam does not change.
B)The intensity of the beam doubles.
C)The energy of individual photons does not change.
D)The energy of individual photons doubles.
E)The wavelength of the individual photons doubles.
A)The power in the beam does not change.
B)The intensity of the beam doubles.
C)The energy of individual photons does not change.
D)The energy of individual photons doubles.
E)The wavelength of the individual photons doubles.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
15
If a proton and an electron have the same de Broglie wavelengths,which one is moving faster?
A)the electron
B)the proton
C)They both have the same speed.
A)the electron
B)the proton
C)They both have the same speed.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
16
Two sources emit beams of microwaves.The microwaves from source A have a frequency of 10 GHz,and the ones from source B have a frequency of 20 GHz.This is all we know about the two beams.Which of the following statements about these beams are correct? (There could be more than one correct choice. )
A)Beam B carries twice as many photons per second as beam A.
B)A photon in beam B has twice the energy of a photon in beam A.
C)The intensity of beam B is twice as great as the intensity of beam A.
D)A photon in beam B has the same energy as a photon in beam A.
E)None of the above statements are true.
A)Beam B carries twice as many photons per second as beam A.
B)A photon in beam B has twice the energy of a photon in beam A.
C)The intensity of beam B is twice as great as the intensity of beam A.
D)A photon in beam B has the same energy as a photon in beam A.
E)None of the above statements are true.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
17
Increasing the brightness of a beam of light without changing its color will increase
A)the number of photons per second traveling in the beam.
B)the energy of each photon.
C)the speed of the photons.
D)the frequency of the light.
E)the wavelength of the photons.
A)the number of photons per second traveling in the beam.
B)the energy of each photon.
C)the speed of the photons.
D)the frequency of the light.
E)the wavelength of the photons.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
18
A photon of blue light and a photon of red light are traveling in vacuum.The photon of blue light
A)has a smaller wavelength than a photon of red light and travels with the same speed.
B)has a smaller wavelength than a photon of red light and travels with a greater speed.
C)has a longer wavelength than a photon of red light and travels with the same speed.
D)has a longer wavelength than a photon of red light and travels with a greater speed.
A)has a smaller wavelength than a photon of red light and travels with the same speed.
B)has a smaller wavelength than a photon of red light and travels with a greater speed.
C)has a longer wavelength than a photon of red light and travels with the same speed.
D)has a longer wavelength than a photon of red light and travels with a greater speed.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
19
Light of a given wavelength is used to illuminate the surface of a metal,however,no photoelectrons are emitted.In order to cause electrons to be ejected from the surface of this metal you should
A)use light of a longer wavelength.
B)use light of a shorter wavelength.
C)use light of the same wavelength but increase its intensity.
D)use light of the same wavelength but decrease its intensity.
A)use light of a longer wavelength.
B)use light of a shorter wavelength.
C)use light of the same wavelength but increase its intensity.
D)use light of the same wavelength but decrease its intensity.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
20
Monochromatic light is incident on a metal surface,and the ejected electrons give rise to a current in the circuit shown in the figure.The maximum kinetic energy of the ejected electrons is determined by applying a reverse ('stopping')potential,sufficient to reduce the current in the ammeter to zero.If the intensity of the incident light is increased,how will the required stopping potential change? 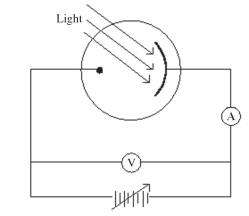
A)It will remain unchanged.
B)It will increase.
C)It will decrease.

A)It will remain unchanged.
B)It will increase.
C)It will decrease.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
21
What is the wavelength of a 6.32-eV photon? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)197 nm
B)167 nm
C)216 nm
D)234 nm
A)197 nm
B)167 nm
C)216 nm
D)234 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
22
Certain planes of a crystal of halite have a spacing of 0.399 nm.The crystal is irradiated by a beam of x-rays.First order constructive interference occurs when the beam makes an angle of 20° with the planes of the crystal surface.What is the wavelength of the x-rays?
A)0.14 nm
B)0.17 nm
C)0.21 nm
D)0.24 nm
E)0.27 nm
A)0.14 nm
B)0.17 nm
C)0.21 nm
D)0.24 nm
E)0.27 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
23
What is the photon energy of red light having a wavelength of 6.40 × 102 nm? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)1.13 × 10-19 J
B)1.31 × 10-19 J
C)3.11 × 10-19 J
D)1.94 × 10-19 J
A)1.13 × 10-19 J
B)1.31 × 10-19 J
C)3.11 × 10-19 J
D)1.94 × 10-19 J

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
24
What is the frequency of the most intense radiation from an object with temperature 100°C? The constant in Wien's law is 0.0029 m ∙ K.(c = 3.0 × 108 m/s)
A)2.9 × 10-5 Hz
B)3.9 × 1013 Hz
C)1.0 × 1013 Hz
D)1.0 × 1011 Hz
A)2.9 × 10-5 Hz
B)3.9 × 1013 Hz
C)1.0 × 1013 Hz
D)1.0 × 1011 Hz

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
25
If the maximum possible accuracy in measuring the velocity of a particle increases,the maximum possible accuracy in measuring its position will
A)increase.
B)decrease.
C)not be affected.
A)increase.
B)decrease.
C)not be affected.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
26
If the maximum possible accuracy in measuring the position of a particle increases,the maximum possible accuracy in measuring its velocity will
A)increase.
B)decrease.
C)not be affected.
A)increase.
B)decrease.
C)not be affected.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
27
What frequency of electromagnetic radiation has photons of energy 4.7 × 10-25 J? (h = 6.626 × 10-34 J ∙ s)
A)710 kHz
B)4.7 MHz
C)710 MHz
D)1.4 GHz
A)710 kHz
B)4.7 MHz
C)710 MHz
D)1.4 GHz

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
28
A proton and an electron are both accelerated to the same final kinetic energy.If λp is the de Broglie wavelength of the proton and λe is the de Broglie wavelength of the electron,then
A)λp > λe.
B)λp = λe.
C)λp < λe.
A)λp > λe.
B)λp = λe.
C)λp < λe.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
29
What is the surface temperature of a star,if its radiation peak occurs at a frequency of 1.06 × 1015 Hz? (c = 3.00 × 108 m/s,and the constant in Wien's law is 0.00290 m ∙ K)
A)17,000 K
B)14,500 K
C)19,000 K
D)20,400 K
E)10,200 K
A)17,000 K
B)14,500 K
C)19,000 K
D)20,400 K
E)10,200 K

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
30
The surface temperature of the star is 6000 K.At what wavelength is its light output a maximum? The constant in Wien's law is 0.00290 m ∙ K.
A)850 nm
B)907 nm
C)311 nm
D)483 nm
E)502 nm
A)850 nm
B)907 nm
C)311 nm
D)483 nm
E)502 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
31
What is the energy (in eV)of an optical photon of frequency 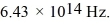
(h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)2.66 eV
B)1.62 eV
C)1.94 eV
D)3.27 eV
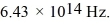
(h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)2.66 eV
B)1.62 eV
C)1.94 eV
D)3.27 eV

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
32
Protons are being accelerated in a particle accelerator.When the speed of the protons is doubled,their de Broglie wavelength will
A)increase by a factor of 4.
B)increase by a factor of 2.
C)decrease by a factor of 2.
D)increase by a factor of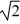 .
.
E)decrease by a factor of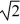 .
.
A)increase by a factor of 4.
B)increase by a factor of 2.
C)decrease by a factor of 2.
D)increase by a factor of
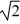 .
.E)decrease by a factor of
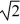 .
.
Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
33
What is the wavelength of the most intense light emitted by a giant star of surface temperature 5000 K? The constant in Wien's law is 0.00290 m ∙ K.
A)576 nm
B)578 nm
C)580 nm
D)582 nm
A)576 nm
B)578 nm
C)580 nm
D)582 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
34
The lattice spacing of the principal Bragg planes in sodium chloride is 0.282 nm.For what wavelength of x-rays will the first order reflected beam diffract at 55° with respect to the normal to the crystal planes?
A)0.323 nm
B)0.530 nm
C)0.662 nm
D)0.150 nm
E)0.462 nm
A)0.323 nm
B)0.530 nm
C)0.662 nm
D)0.150 nm
E)0.462 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
35
Each photon in a beam of light has an energy of 4.20 eV.What is the wavelength of this light? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)321 nm
B)103 nm
C)296 nm
D)412 nm
E)420 nm
A)321 nm
B)103 nm
C)296 nm
D)412 nm
E)420 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
36
Certain planes of a crystal of halite have a spacing of 0.399 nm.The crystal is irradiated by a beam of x-rays.First order constructive interference occurs when the beam makes an angle of 20° with the planes of the crystal surface.What angle does the beam make with the crystal planes for second order constructive?
A)37°
B)40°
C)43°
D)46°
E)49°
A)37°
B)40°
C)43°
D)46°
E)49°

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
37
If the sunlight from a star peaks at a wavelength of 0.55 µm,what temperature does this imply for the surface of that star? The constant in Wien's law is 0.00290 m ∙ K.
A)9500 K
B)5300 K
C)2500 K
D)25,000 K
E)15,000 K
A)9500 K
B)5300 K
C)2500 K
D)25,000 K
E)15,000 K

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
38
A proton and an electron are both accelerated to the same final speed.If λp is the de Broglie wavelength of the proton and λe is the de Broglie wavelength of the electron,then
A)λp > λe.
B)λp = λe.
C)λp < λe.
A)λp > λe.
B)λp = λe.
C)λp < λe.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
39
A crystal is irradiated with x-rays with a wavelength of 0.120 nm.The atomic planes in the crystal are separated by 0.21 nm.At what angles of incidence with respect to the normal will the x-rays reflect from the crystal?
A)73°,55°,31°
B)only 55°
C)only 73° and 31°
D)only 73°
A)73°,55°,31°
B)only 55°
C)only 73° and 31°
D)only 73°

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
40
How much energy is carried by a photon of light having frequency 110 GHz? (h = 6.626 × 10-34 J ∙ s)
A)1.1 × 10-20 J
B)1.4 × 10-22 J
C)7.3 × 10-23 J
D)1.3 × 10-25 J
A)1.1 × 10-20 J
B)1.4 × 10-22 J
C)7.3 × 10-23 J
D)1.3 × 10-25 J

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
41
An 84-kW AM radio station broadcasts at 1000 kHz.How many photons are emitted each second by the transmitting antenna? (h = 6.626 × 10-34 J ∙ s)
A)1.3 ×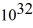
B)2.9 ×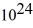
C)6.3 ×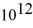
D)1.4 ×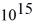
A)1.3 ×
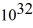
B)2.9 ×
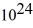
C)6.3 ×
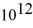
D)1.4 ×
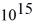

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
42
A metal has a work function of 4.50 eV.Find the maximum kinetic energy of the photoelectrons if light of wavelength 250 nm shines on the metal.(1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)0.00 eV
B)0.37 eV
C)0.47 eV
D)0.53 eV
A)0.00 eV
B)0.37 eV
C)0.47 eV
D)0.53 eV

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
43
The work function of a particular metal is 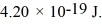
What is the photoelectric cutoff (threshold)wavelength for this metal? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)473 nm
B)308 nm
C)393 nm
D)554 nm
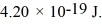
What is the photoelectric cutoff (threshold)wavelength for this metal? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)473 nm
B)308 nm
C)393 nm
D)554 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
44
What is the cutoff (threshold)frequency for a metal surface that has a work function of 5.42 eV? (1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J ∙ s)
A)1.31 ×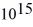 Hz
Hz
B)2.01 ×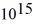 Hz
Hz
C)3.01 ×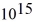 Hz
Hz
D)5.02 ×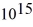 Hz
Hz
E)6.04 ×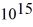 Hz
Hz
A)1.31 ×
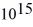 Hz
HzB)2.01 ×
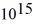 Hz
HzC)3.01 ×
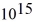 Hz
HzD)5.02 ×
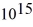 Hz
HzE)6.04 ×
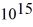 Hz
Hz
Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
45
A photocathode whose work function is 2.9 eV is illuminated with white light that has a continuous wavelength band from 400 nm to 700 nm.What is the range of the wavelength band in this white light illumination for which photoelectrons are not produced? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)430 nm to 700 nm
B)400 nm to 480 nm
C)430 nm to 480 nm
D)400 nm to 430 nm
E)480 nm to 700 nm
A)430 nm to 700 nm
B)400 nm to 480 nm
C)430 nm to 480 nm
D)400 nm to 430 nm
E)480 nm to 700 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
46
Gamma rays are photons with very high energy.How many visible-light photons with a wavelength of 500 nm would you need to equal the energy of a gamma-ray photon with energy 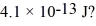
A)1.0 × 106
B)1.4 × 108
C)6.2 × 109
D)3.9 × 103
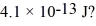
A)1.0 × 106
B)1.4 × 108
C)6.2 × 109
D)3.9 × 103

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
47
If the work function of a metal surface is 2.20 eV,what frequency of incident light would give a maximum kinetic energy of 0.25 eV to the photoelectrons ejected from this surface? (1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J ∙ s)
A)2.05 × 1014 Hz
B)1.02 × 1014 Hz
C)2.50 × 1014 Hz
D)3.53 × 1014 Hz
E)5.92 × 1014 Hz
A)2.05 × 1014 Hz
B)1.02 × 1014 Hz
C)2.50 × 1014 Hz
D)3.53 × 1014 Hz
E)5.92 × 1014 Hz

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
48
A laser emits a pulse of light that lasts 10 ns.The light has a wavelength of 690 nm,and each pulse has an energy of 480 mJ.How many photons are emitted in each pulse? (c = 3.0 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)1.7 × 1018
B)2.1 ×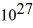
C)2.6 ×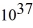
D)3.1 ×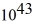
A)1.7 × 1018
B)2.1 ×
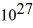
C)2.6 ×
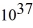
D)3.1 ×
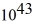

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
49
Light with a wavelength of 310 nm is incident on a metal that has a work function of 3.80 eV.What is the maximum kinetic energy that a photoelectron ejected in this process can have? (1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)0.62 × 10-19 J
B)0.21 × 10-19 J
C)0.36 × 10-19 J
D)0.48 × 10-19 J
E)0.33 × 10-19 J
A)0.62 × 10-19 J
B)0.21 × 10-19 J
C)0.36 × 10-19 J
D)0.48 × 10-19 J
E)0.33 × 10-19 J

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
50
A laser pulse of duration 25 ms has a total energy of 1.4 J.If the wavelength of this radiation is 567 nm,how many photons are emitted in one pulse? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)4.0 × 1018
B)9.9 × 1019
C)4.8 × 1019
D)1.6 × 1017
E)3.2 × 1017
A)4.0 × 1018
B)9.9 × 1019
C)4.8 × 1019
D)1.6 × 1017
E)3.2 × 1017

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
51
What is the longest wavelength of electromagnetic radiation that will eject photoelectrons from sodium metal for which the work function is 2.28 eV? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)580 nm
B)499 nm
C)633 nm
D)668 nm
E)545 nm
A)580 nm
B)499 nm
C)633 nm
D)668 nm
E)545 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
52
For what wavelength does a 100-mW laser beam deliver 1.6 × 1017 photons in one second? (c = 3.0 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)320 nm
B)330 nm
C)340 nm
D)350 nm
A)320 nm
B)330 nm
C)340 nm
D)350 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
53
What is the longest wavelength of light that can cause photoelectron emission from a metal that has a work function of 2.20 eV? (1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)417 nm
B)257 nm
C)344 nm
D)565 nm
E)610 nm
A)417 nm
B)257 nm
C)344 nm
D)565 nm
E)610 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
54
Gamma rays are photons with very high energy.What is the wavelength of a gamma-ray photon with energy 7.7 × 10-13 J? (c = 3.0 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)2.6 × 10-13 m
B)3.9 × 10-13 m
C)3.1 × 10-13 m
D)3.5 × 10-13 m
A)2.6 × 10-13 m
B)3.9 × 10-13 m
C)3.1 × 10-13 m
D)3.5 × 10-13 m

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
55
When a metal is illuminated by light,photoelectrons are observed provided that the wavelength of the light is less than 520 nm.What is the metal's work function? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)2.4 eV
B)2.6 eV
C)2.8 eV
D)3.0 eV
A)2.4 eV
B)2.6 eV
C)2.8 eV
D)3.0 eV

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
56
A helium-neon laser emits light at 632.8 nm.If the laser emits 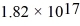
Photons/second,what is its power output in mW? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)57.2 mW
B)28.6 mW
C)37.2 mW
D)45.7 mW
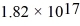
Photons/second,what is its power output in mW? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)57.2 mW
B)28.6 mW
C)37.2 mW
D)45.7 mW

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
57
A beam of light with a frequency range from 3.01 × 1014 Hz to 6.10 × 1014 Hz is incident on a metal surface.If the work function of the metal surface is 2.20 eV,what is the maximum kinetic energy of photoelectrons ejected from this surface? (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)0.33 eV
B)0.21 eV
C)0.42 eV
D)0.16 eV
E)0.48 eV
A)0.33 eV
B)0.21 eV
C)0.42 eV
D)0.16 eV
E)0.48 eV

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
58
The work function of a certain metal is 1.90 eV.What is the longest wavelength of light that can cause photoelectron emission from this metal? (1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)231 nm
B)14.0 nm
C)62.4 nm
D)344 nm
E)654 nm
A)231 nm
B)14.0 nm
C)62.4 nm
D)344 nm
E)654 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
59
When it is struck by 240-nm photons,a metal ejects electrons with a maximum kinetic energy of 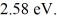
What is the work function of this material? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 16.0 × 10-19 J)
A)2.60 eV
B)2.18 eV
C)3.02 eV
D)3.43 eV
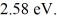
What is the work function of this material? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 16.0 × 10-19 J)
A)2.60 eV
B)2.18 eV
C)3.02 eV
D)3.43 eV

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
60
Light with a frequency of 8.70 × 1014 Hz is incident on a metal that has a work function of 2.80 eV.What is the maximum kinetic energy that a photoelectron ejected in this process can have? (1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J ∙ s)
A)8.7 × 10-19 J
B)3.1 × 10-19 J
C)1.3 × 10-19 J
D)2.4 × 10-19 J
E)4.5 × 10-19 J
A)8.7 × 10-19 J
B)3.1 × 10-19 J
C)1.3 × 10-19 J
D)2.4 × 10-19 J
E)4.5 × 10-19 J

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
61
An electron is moving with the speed of 1780 m/s.What is its de Broglie wavelength? (melectron = 9.11 × 10-31 kg,h = 6.626 × 10-34 J ∙ s)
A)409 nm
B)302 nm
C)205 nm
D)420 nm
E)502 nm
A)409 nm
B)302 nm
C)205 nm
D)420 nm
E)502 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
62
If the de Broglie wavelength of an electron is 2.4 μm,what is the speed of the electron? 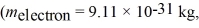
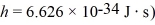
A)3.0 × 102 m/s
B)2.5 × 105 m/s
C)1.7 × 103 m/s
D)8.3 × 106 m/s
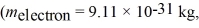
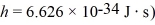
A)3.0 × 102 m/s
B)2.5 × 105 m/s
C)1.7 × 103 m/s
D)8.3 × 106 m/s

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
63
When light of wavelength 350 nm is incident on a metal surface,the stopping potential of the photoelectrons is 0.500 V.What is the maximum kinetic energy of these electrons? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)0.500 eV
B)3.04 eV
C)3.54 eV
D)4.12 eV
A)0.500 eV
B)3.04 eV
C)3.54 eV
D)4.12 eV

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
64
If the momentum of an electron is 1.95 × 10-27 kg ∙ m/s,what is its de Broglie wavelength? (h = 6.626 × 10-34 J ∙ s)
A)340 nm
B)210 nm
C)170 nm
D)420 nm
E)520 nm
A)340 nm
B)210 nm
C)170 nm
D)420 nm
E)520 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
65
What is the wavelength of the matter wave associated with a 0.50-kg ball moving at 25 m/s? (h = 6.626 × 10-34 J ∙ s)
A)3.5 × 10-35 m
B)5.3 × 10-35 m
C)3.5 × 10-33 m
D)5.3 × 10-33 m
A)3.5 × 10-35 m
B)5.3 × 10-35 m
C)3.5 × 10-33 m
D)5.3 × 10-33 m

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
66
After an electron has been accelerated through a potential difference of 0.15 kV,what is its de Broglie wavelength? (e = 1.60 × 10-19 C,melectron = 9.11 × 10-31 kg,h = 6.626 × 10-34 J ∙ s)
A)0.10 nm
B)1.0 nm
C)1.0 mm
D)1.0 cm
A)0.10 nm
B)1.0 nm
C)1.0 mm
D)1.0 cm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
67
A photocathode having a work function of 2.8 eV is illuminated with monochromatic electromagnetic radiation whose photon energy is 4.0 eV.What is the threshold (cutoff)frequency for photoelectron production? (1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J ∙ s)
A)6.8 × 1014 Hz
B)2.9 × 1014 Hz
C)7.7 × 1014 Hz
D)8.6 × 1014 Hz
E)9.7 × 1014 Hz
A)6.8 × 1014 Hz
B)2.9 × 1014 Hz
C)7.7 × 1014 Hz
D)8.6 × 1014 Hz
E)9.7 × 1014 Hz

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
68
If an electron has a wavelength of 0.123 nm,what is its kinetic energy,in electron-volts? This energy is not in the relativistic region.(melectron = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J ∙ s)
A)19.8 eV
B)60.2 eV
C)80.4 eV
D)99.5 eV
E)124 eV
A)19.8 eV
B)60.2 eV
C)80.4 eV
D)99.5 eV
E)124 eV

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
69
When light of wavelength 350 nm is incident on a metal surface,the stopping potential of the photoelectrons is 0.500 V.What is the threshold (cutoff)frequency of this metal? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)3.47 × 1014 Hz
B)3.74 × 1014 Hz
C)4.73 × 1014 Hz
D)7.36 × 1014 Hz
A)3.47 × 1014 Hz
B)3.74 × 1014 Hz
C)4.73 × 1014 Hz
D)7.36 × 1014 Hz

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
70
What is the wavelength of the matter wave associated with an electron moving with a speed of 2.5 × 107 m/s? (melectron = 9.11 × 10-31 kg,h = 6.626 × 10-34 J ∙ s)
A)29 pm
B)35 pm
C)47 pm
D)53 pm
A)29 pm
B)35 pm
C)47 pm
D)53 pm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
71
When it is struck by 240-nm photons,a material having a work function of 2.60 eV emits electrons.What is the maximum kinetic energy of the emitted electrons? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)2.58 eV
B)5.18 eV
C)2.00 eV
D)4.21 eV
A)2.58 eV
B)5.18 eV
C)2.00 eV
D)4.21 eV

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
72
A monochromatic light beam is incident on the surface of a metal having a work function of 2.50 eV.If a 1.0-V stopping potential is required to make the electron current zero,what is the wavelength of light? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)355 nm
B)423 nm
C)497 nm
D)744 nm
A)355 nm
B)423 nm
C)497 nm
D)744 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
73
If an electron has the same de Broglie wavelength as the wavelength of a 390-nm photon in vacuum,what is the speed of the electron? (melectron = 9.11 × 10-31 kg,c = 3.0 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)1,900 m/s
B)2,100 m/s
C)1,700m/s
D)1,500 m/s
E)540 m/s
A)1,900 m/s
B)2,100 m/s
C)1,700m/s
D)1,500 m/s
E)540 m/s

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
74
If the de Broglie wavelength of an electron is 380 nm,what is the speed of this electron? (melectron = 9.11 × 10-31 kg,h = 6.626 × 10-34 J ∙ s)
A)2.0 km/s
B)3.8 km/s
C)1.9 km/s
D)4.1 km/s
E)5.2 km/s
A)2.0 km/s
B)3.8 km/s
C)1.9 km/s
D)4.1 km/s
E)5.2 km/s

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
75
A photocathode having a work function of 2.4 eV is illuminated with monochromatic light whose photon energy is 3.4 eV.What is maximum kinetic energy of the photoelectrons produced? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)1.6 × 10-19 J
B)3.8 × 10-19 J
C)4.4 × 10-19 J
D)4.9 × 10-19 J
E)5.4 × 10-19 J
A)1.6 × 10-19 J
B)3.8 × 10-19 J
C)4.4 × 10-19 J
D)4.9 × 10-19 J
E)5.4 × 10-19 J

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
76
What is the de Broglie wavelength of a ball of mass 200 g moving at 30 m/s? (h = 6.626 × 10-34 J ∙ s)
A)1.1 × 10-34 m
B)2.2 × 10-34 m
C)4.5 × 10-28 m
D)6.7 × 10-27 m
A)1.1 × 10-34 m
B)2.2 × 10-34 m
C)4.5 × 10-28 m
D)6.7 × 10-27 m

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
77
Find the de Broglie wavelength of a 1.30-kg missile moving at 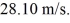
(h = 6.626 × 10-34 J ∙ s)
A)1.81 × 10-35 m
B)2.05 × 10-35 m
C)2.28 × 10-35 m
D)2.57 × 10-35 m
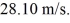
(h = 6.626 × 10-34 J ∙ s)
A)1.81 × 10-35 m
B)2.05 × 10-35 m
C)2.28 × 10-35 m
D)2.57 × 10-35 m

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
78
A person of mass 50 kg has a de Broglie wavelength of 4.4 × 10-36 m while jogging.How fast is she running? (h = 6.626 × 10-34 J ∙ s)
A)2.0 m/s
B)3.0 m/s
C)4.0 m/s
D)5.0 m/s
A)2.0 m/s
B)3.0 m/s
C)4.0 m/s
D)5.0 m/s

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
79
A photocathode that has a work function of 2.4 eV is illuminated with monochromatic light having photon energy 3.5 eV.What is the wavelength of this light? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)350 nm
B)330 nm
C)300 nm
D)380 nm
E)410 nm
A)350 nm
B)330 nm
C)300 nm
D)380 nm
E)410 nm

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
80
When light of wavelength 350 nm is incident on a metal surface,the stopping potential of the photoelectrons is measured to be 0.500 V.What is the work function of this metal? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)0.500 eV
B)3.05 eV
C)3.54 eV
D)4.12 eV
A)0.500 eV
B)3.05 eV
C)3.54 eV
D)4.12 eV

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck


