Deck 17: Phases and Phase Changes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 17: Phases and Phase Changes
1
In kinetic theory, a gas is modeled as being made up of a large number of molecules which bounce from the walls of the container.
True
2
When both the pressure and the absolute temperature of an ideal gas are increased each by a factor of two, the volume occupied by the gas will
A) increase by a factor of four.
B) increase by a factor of two.
C) not change.
D) decrease by a factor of two.
E) decrease by a factor of four.
A) increase by a factor of four.
B) increase by a factor of two.
C) not change.
D) decrease by a factor of two.
E) decrease by a factor of four.
not change.
3
The internal energy of an ideal gas does not depend on the pressure.
True
4
When comparing two gases at the same temperature, the gas with a higher molecular weight has more internal energy per mole.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
The pressure in a gas is the result of the momentum transfer that occurs when the molecules bounce from the walls of the container.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
According to kinetic theory, the temperature of a gas is determined by the average kinetic energy of its molecules. What would happen if the gas were in a container that was moving with a high velocity?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
You have had something simmering in a covered pot on the stove and then turn off the heat. Some time later you return to the pot and find that you have trouble lifting the lid. Why?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
Explain why, when someone across the room pierces a can of coffee with a can opener, you hear the sound of the can being pierced before you smell the aroma of the coffee.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
On a cold winter day, you turn up the thermostat in your house. Assume that your house is well sealed and that no air enters or leaves the house. When the air temperature in your house rises a short while later, what statement is correct regarding the air pressure?
A) The air pressure is higher at the higher temperature.
B) The air pressure is lower at the higher temperature.
C) The air pressure does not change at the higher temperature.
D) The air pressure increases at first but then decreases again as the temperature approaches its higher value.
E) The air pressure decreases at first but then increases again as the temperature approaches its lower value.
A) The air pressure is higher at the higher temperature.
B) The air pressure is lower at the higher temperature.
C) The air pressure does not change at the higher temperature.
D) The air pressure increases at first but then decreases again as the temperature approaches its higher value.
E) The air pressure decreases at first but then increases again as the temperature approaches its lower value.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
State Boyle's law.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
In the deep ocean trenches, there are hydrothermal vents, which discharge very hot water. In one such vent, the temperature of the water is 300°C. Why does this water not boil?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
When you are standing by an air conditioning vent in an indoor swimming pool area, you feel a lot cooler if you are wet than if you are dry. Why?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
State Charles's law.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
On a hot summer day, you turn the thermostat in your house way down. Assume that your house is well sealed and that no air enters or leaves the house. When the air temperature in your house falls a short while later, what statement is correct regarding the air pressure?
A) The air pressure is higher at the lower temperature.
B) The air pressure is lower at the lower temperature.
C) The air pressure does not change at the lower temperature.
D) The air pressure increases at first but then decreases again as the temperature approaches its higher value.
E) The air pressure decreases at first but then increases again as the temperature approaches its lower value.
A) The air pressure is higher at the lower temperature.
B) The air pressure is lower at the lower temperature.
C) The air pressure does not change at the lower temperature.
D) The air pressure increases at first but then decreases again as the temperature approaches its higher value.
E) The air pressure decreases at first but then increases again as the temperature approaches its lower value.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
In tropical areas of the world where refrigeration is not readily available, people store water in unglazed clay pots. This makes the water stay at a cool temperature, much cooler than the ambient temperature. How is that possible?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
Ice melts if enough pressure is applied to it.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
When comparing two gases at the same temperature, the molecules of the gas with the smaller molecular weight have the higher rms speed.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
What is the Maxwell distribution?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
When a spray can is being used, it feels cooler than when it was first picked up. Why?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
The internal energy of an ideal gas does not depend on the volume.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
The point in the phase diagram where the vapor pressure curve ends is called the
A) critical point.
B) triple point.
C) melting point.
D) boiling point.
E) double point.
A) critical point.
B) triple point.
C) melting point.
D) boiling point.
E) double point.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
The point in the phase diagram where the fusion curve, the vapor pressure curve, and the sublimation curve join is called the
A) critical point.
B) triple point.
C) melting point.
D) boiling point.
E) double point.
A) critical point.
B) triple point.
C) melting point.
D) boiling point.
E) double point.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
A sample of an ideal gas is slowly compressed to one-half its original volume with no change in temperature. What happens to the average speed of the molecules in the sample?
A) It does not change.
B) It quadruples.
C) It doubles.
D) It halves.
E) cannot be determined without more information
A) It does not change.
B) It quadruples.
C) It doubles.
D) It halves.
E) cannot be determined without more information

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
Two containers of equal volume each hold samples of the same ideal gas. Container A has twice as many molecules as container B. If the gas pressure is the same in the two containers, the correct statement regarding the absolute temperatures TA and TB in containers A and B, respectively, is
A) TA = TB .
B) TA = 2TB .
C) TA =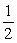 TB .
TB .
D) TA =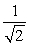 TB .
TB .
E) TA =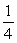 TB .
TB .
A) TA = TB .
B) TA = 2TB .
C) TA =
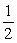 TB .
TB .D) TA =
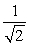 TB .
TB .E) TA =
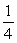 TB .
TB .
Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
A liquid boils when its vapor pressure
A) equals the equilibrium vapor pressure.
B) equals the external pressure.
C) exceeds the external pressure.
D) is between the equilibrium vapor pressure and the external pressure.
E) None of the other choices is correct.
A) equals the equilibrium vapor pressure.
B) equals the external pressure.
C) exceeds the external pressure.
D) is between the equilibrium vapor pressure and the external pressure.
E) None of the other choices is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
When a vapor condenses
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
Steam burns can be much more severe than burns caused by boiling water. The reason for this is that
A) steam can go through the clothes.
B) steam delivers more energy to the skin than boiling water does.
C) steam immediately penetrates the skin and then burns from the inside out.
D) all of the above
E) none of the above
A) steam can go through the clothes.
B) steam delivers more energy to the skin than boiling water does.
C) steam immediately penetrates the skin and then burns from the inside out.
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following best explains why sweating is important to humans in maintaining suitable body temperature?
A) Functioning of the sweat gland absorbs energy that otherwise would go into heating the body.
B) The high specific heat of water on the skin absorbs heat from the body.
C) Evaporation of moisture from the skin extracts heat from the body.
D) Moisture on the skin increases thermal conductivity, thereby allowing heat to flow out of the body more effectively.
E) None of the other choices is correct.
A) Functioning of the sweat gland absorbs energy that otherwise would go into heating the body.
B) The high specific heat of water on the skin absorbs heat from the body.
C) Evaporation of moisture from the skin extracts heat from the body.
D) Moisture on the skin increases thermal conductivity, thereby allowing heat to flow out of the body more effectively.
E) None of the other choices is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
The heat required to change a substance from the liquid to the vapor state is referred to as the
A) heat of fusion.
B) heat of vaporization.
C) heat of evaporation.
D) heat of condensation.
E) heat of melting.
A) heat of fusion.
B) heat of vaporization.
C) heat of evaporation.
D) heat of condensation.
E) heat of melting.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
A sample of an ideal gas is heated and its Kelvin temperature doubles. What happens to the average speed of the molecules in the sample?
A) It does not change.
B) It halves.
C) It doubles.
D) It is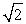 times as large.
times as large.
E) It is 1/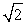 times smaller.
times smaller.
A) It does not change.
B) It halves.
C) It doubles.
D) It is
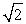 times as large.
times as large.E) It is 1/
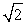 times smaller.
times smaller.
Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
When we say that a material sample has reached its elastic limit, we mean that
A) if any further stress is applied, the sample will break.
B) if any further stress is applied, the deformation of the sample will not be reversible.
C) the deformation no longer follows Hooke's law.
D) the material will continue deforming with no further additional stress.
E) None of the other choices is correct.
A) if any further stress is applied, the sample will break.
B) if any further stress is applied, the deformation of the sample will not be reversible.
C) the deformation no longer follows Hooke's law.
D) the material will continue deforming with no further additional stress.
E) None of the other choices is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
When a liquid evaporates
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
When a solid melts
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
When a liquid freezes
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
In a city high in the mountains, such as Denver, CO, water boils
A) at the same temperature as it does at sea level.
B) at a higher temperature than it does at sea level.
C) at a lower temperature than it does at sea level.
D) at a higher temperature than it does at sea level in the morning but at a lower temperature than it does at sea level the rest of the day.
E) at a lower temperature than it does at sea level in the morning but at a higher temperature than it does at sea level the rest of the day.
A) at the same temperature as it does at sea level.
B) at a higher temperature than it does at sea level.
C) at a lower temperature than it does at sea level.
D) at a higher temperature than it does at sea level in the morning but at a lower temperature than it does at sea level the rest of the day.
E) at a lower temperature than it does at sea level in the morning but at a higher temperature than it does at sea level the rest of the day.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
The internal energy of an ideal gas depends on
A) its pressure.
B) its volume.
C) its temperature.
D) its temperature and pressure.
E) its temperature, pressure, and volume.
A) its pressure.
B) its volume.
C) its temperature.
D) its temperature and pressure.
E) its temperature, pressure, and volume.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
Phase changes occur
A) as the temperature decreases.
B) as the temperature increases.
C) as the temperature remains the same.
D) all of the above
E) none of the above
A) as the temperature decreases.
B) as the temperature increases.
C) as the temperature remains the same.
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
Phase equilibrium between the gaseous and liquid states describes the situation when
A) no more molecules leave the liquid and enter the gas.
B) no more molecules leave the gas and enter the liquid.
C) as many molecules go from liquid to gas as from gas to liquid.
D) the liquid is boiling.
E) None of the other choices is correct.
A) no more molecules leave the liquid and enter the gas.
B) no more molecules leave the gas and enter the liquid.
C) as many molecules go from liquid to gas as from gas to liquid.
D) the liquid is boiling.
E) None of the other choices is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
La Paz, Bolivia, is at an altitude of 3,650 meters above sea level. In La Paz, water will boil
A) at 100°C.
B) below 100°C.
C) above 100°C.
D) There is not enough information to answer this question.
A) at 100°C.
B) below 100°C.
C) above 100°C.
D) There is not enough information to answer this question.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
The heat required to change a substance from the solid to the liquid state is referred to as the
A) heat of fusion.
B) heat of vaporization.
C) heat of melting.
D) heat of freezing.
E) heat of condensation.
A) heat of fusion.
B) heat of vaporization.
C) heat of melting.
D) heat of freezing.
E) heat of condensation.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
A container of negligible heat capacity has in it 456 g of ice at -25.0°C. Heat is supplied to the container at the rate of 1000 J/min. How long will it take before the temperature starts to rise above 0°C?
The specific heat of ice is 2090 J/(kg K) and the latent heat of fusion of water is
33.5 × 104 J/kg.
The specific heat of ice is 2090 J/(kg K) and the latent heat of fusion of water is
33.5 × 104 J/kg.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
A container of negligible heat capacity has in it 456 g of ice at -25.0°C. Heat is supplied to the container at the rate of 1000 J/min. After how long will the ice start to melt, assuming all of the ice has the same temperature?
The specific heat of ice is 2090 J/(kg K) and the latent heat of fusion of water is 33.5 × 104 J/kg.
The specific heat of ice is 2090 J/(kg K) and the latent heat of fusion of water is 33.5 × 104 J/kg.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
What is the rms speed of a helium atom:
(a) at 5. K?
(b) at -196.° C (the boiling point of N2)?
(c) at 100.° C?
(a) at 5. K?
(b) at -196.° C (the boiling point of N2)?
(c) at 100.° C?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
What is the temperature of 3.0 moles of gas at a pressure of 250 kPa held in a volume of 15 L?
A) 150 K
B) 200 K
C) 250 K
D) 300 K
E) 350 K
A) 150 K
B) 200 K
C) 250 K
D) 300 K
E) 350 K

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
An automobile tire has a volume of 0.0185 m3. If the pressure in the tire is 500 kPa and the temperature is 298 K, how many molecules are there inside the tire?
A) 2.25 × 1023
B) 2.25 × 1024
C) 3.25 × 1023
D) 3.25 × 1024
E) 3.25 × 1025
A) 2.25 × 1023
B) 2.25 × 1024
C) 3.25 × 1023
D) 3.25 × 1024
E) 3.25 × 1025

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
A laboratory vacuum pump can reduce the pressure to 1.0 × 10-7 Pa. If the volume of the chamber is 0.500 m3 and the temperature is 27°C, how many molecules are left inside the chamber?
A) 1.2 × 1013
B) 2.4 × 1013
C) 1.2 × 1012
D) 2.4 × 1012
E) 1.2 × 1014
A) 1.2 × 1013
B) 2.4 × 1013
C) 1.2 × 1012
D) 2.4 × 1012
E) 1.2 × 1014

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
Your lungs hold 4.2 L of air at a temperature of 27°C and a pressure of 101.3 kPa. How many moles of air do your lungs hold?
A) 0.15 moles
B) 0.17 moles
C) 0.19 moles
D) 0.21 moles
E) 0.23 moles
A) 0.15 moles
B) 0.17 moles
C) 0.19 moles
D) 0.21 moles
E) 0.23 moles

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
Your lungs hold 4.2 L of air at a pressure of 101.3 kPa. If you are holding your breath and dive into a pool to a depth of 5.0 m below the surface of the water, what is the volume of the air in your lungs assuming that the temperature remains the same?
A) 2.8 L
B) 2.9 L
C) 3.2 L
D) 3.3 L
E) 3.5 L
A) 2.8 L
B) 2.9 L
C) 3.2 L
D) 3.3 L
E) 3.5 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
Using K = 3/2 kT, what is the average kinetic energy of a He atom at:
(a) 10. K?
(b) -196.°C?
(c) 100.°C?
(a) 10. K?
(b) -196.°C?
(c) 100.°C?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
Two moles of gas are at STP. If the temperature changes to 47.° C and the pressure decreases to half of what it was, what volume do the two moles now occupy?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
A vertical cylindrical cylinder, closed at the bottom end, contains 0.0100 moles of gas. It is fitted at the top with a piston, which can move freely. The mass of the piston is 14.0 kg and the initial height of the piston above the bottom of the cylinder is 25 cm. What is the temperature of the gas?
A) 290 K
B) 413 K
C) 3620 K
D) 405 K
E) 500 K
A) 290 K
B) 413 K
C) 3620 K
D) 405 K
E) 500 K

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
A refrigerator has an interior volume of 0.500 m3. The temperature inside the refrigerator in 282 K, and the pressure is 101 kPa. If the molecular weight of air is 29 g/mol, what is the mass of air inside the refrigerator?
A) 625 g
B) 513 g
C) 447 g
D) 329 g
E) 243 g
A) 625 g
B) 513 g
C) 447 g
D) 329 g
E) 243 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
How many molecules are in:
(a) 1.0 cm3 of AIR at STP?
(b) 1.0 cm3 of HELIUM at STP?
(a) 1.0 cm3 of AIR at STP?
(b) 1.0 cm3 of HELIUM at STP?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
A 20.0-L pressure vessel holds 2.00 moles of oxygen at 30°C. What is the pressure inside the vessel?
A) 101 Pa
B) 101 kPa
C) 1.01 × 106 Pa
D) 2.52 × 106 Pa
E) 252 kPa
A) 101 Pa
B) 101 kPa
C) 1.01 × 106 Pa
D) 2.52 × 106 Pa
E) 252 kPa

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
On a cold day, you take in 4.2 L of air into your lungs at a temperature of 0°C. If you hold your breath until the temperature of the air in your lungs reaches 37°C, what is the volume of the air in your lungs at that point, assuming the pressure does not change?
A) 4.2 L
B) 4.4 L
C) 4.6 L
D) 4.8 L
E) 5.0 L
A) 4.2 L
B) 4.4 L
C) 4.6 L
D) 4.8 L
E) 5.0 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
A 600-g piece of iron at 100°C is dropped into a calorimeter of negligible heat capacity containing 100 g of ice at 0°C and 120 g of water, also at 0°C. What is the final temperature of the system?
The specific heat of iron is 448 J/(kg K) and the latent heat of fusion of water is
33.5 × 104 J/kg.
The specific heat of iron is 448 J/(kg K) and the latent heat of fusion of water is
33.5 × 104 J/kg.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
A balloon containing 2.0 m3 of hydrogen gas rises from a location at which the temperature is 22°C and the pressure is 101 kPa to a location where the temperature is -39°C and the pressure is 20 kPa. If the balloon is free to expand so that the pressure of the gas inside is equal to the ambient pressure, what is the new volume of the balloon?
A) 4.0 m3
B) 6.0 m3
C) 8.0 m3
D) 10 m3
E) 12 m3
A) 4.0 m3
B) 6.0 m3
C) 8.0 m3
D) 10 m3
E) 12 m3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
A car starts out when the air temperature is 288 K. The air pressure in the tires is 500 kPa. After driving a while, the temperature of the air in the tires increases to 298 K. What is the pressure in the tires at that point, assuming the volume remains constant?
A) 129 kPa
B) 483 kPa
C) 507 kPa
D) 517 kPa
E) 532 kPa
A) 129 kPa
B) 483 kPa
C) 507 kPa
D) 517 kPa
E) 532 kPa

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
The melting point of aluminum is 660°C, the latent heat of fusion is 4.00 × 105 J/kg, and its specific heat is 900 J/(kg K). 300 kJ of heat are added to 442 g of aluminum at 100°C, what is the final state of the system?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
A vertical cylindrical cylinder, closed at the bottom end, is fitted at the top with a piston, which can move freely. The mass of the piston is 10.0 kg and the initial height of the piston above the bottom of the cylinder is 25 cm. A mass of 8 kg is placed on the piston. What is the resulting height of the piston, assuming that the temperature is kept constant?
A) 12 cm
B) 13 cm
C) 14 cm
D) 15 cm
E) 16 cm
A) 12 cm
B) 13 cm
C) 14 cm
D) 15 cm
E) 16 cm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
The rms speed of a certain sample of carbon dioxide molecules, with a molecular weight of 44 g/mole is 396 m/s. What is the rms speed of water molecules, with a molecular weight of 18 g/mol at the same temperature?
A) 253 m/s
B) 387 m/s
C) 421 m/s
D) 506 m/s
E) 619 m/s
A) 253 m/s
B) 387 m/s
C) 421 m/s
D) 506 m/s
E) 619 m/s

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
A 1200-kg car is being raised with a constant acceleration of 2.53 m/s2 by a crane, using a 20-m long steel cable, 1.5 cm in diameter. Young's modulus for steel is 20 × 1010 N/m2. What is the change in length of the cable?
A) 8.4 mm
B) 8.4 cm
C) 4.9 mm
D) 4.9 cm
E) 4.9 m
A) 8.4 mm
B) 8.4 cm
C) 4.9 mm
D) 4.9 cm
E) 4.9 m

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
Opposing shearing forces of 3000 N are applied to the 4.00 cm × 5.00 cm faces of a 3.00 cm × 4.00 cm × 5.00 cm steel block. By what amount does the block become slanted? The shear modulus for steel is 8.10 × 1010 N/m2.
A) 1.54 × 10-6 m
B) 5.56 × 10-7 m
C) 9.88 × 10-7 m
D) 3.08 × 10-6 m
E) 6.98 × 10-6 m
A) 1.54 × 10-6 m
B) 5.56 × 10-7 m
C) 9.88 × 10-7 m
D) 3.08 × 10-6 m
E) 6.98 × 10-6 m

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
At what temperature is the rms speed of hydrogen molecules, which have a molecular weight of 2.02 g/mole, equal to 2000 m/s?
A) 17.0°C
B) 34.0°C
C) 51.0°C
D) 68.0°C
E) 72.0°C
A) 17.0°C
B) 34.0°C
C) 51.0°C
D) 68.0°C
E) 72.0°C

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
What is the internal energy in 2.7 moles of hydrogen at 300 K? As an approximation, treat the hydrogen gas as if it was monatomic.
A) 10 kJ
B) 12 kJ
C) 14 kJ
D) 16 kJ
E) 18 kJ
A) 10 kJ
B) 12 kJ
C) 14 kJ
D) 16 kJ
E) 18 kJ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
A 50-kg air conditioner is placed on top of a concrete pad with a cross-sectional area of 3.0 m2 and 20 cm thick. What is the change in thickness of the pad? Young's modulus for concrete is 2.3 × 1010 N/m2.
A) 1.4 × 10-9 m
B) 2.8 × 10-9 m
C) 1.4 × 10-8 m
D) 1.4 × 10-7 m
E) 2.8 × 10-8 m
A) 1.4 × 10-9 m
B) 2.8 × 10-9 m
C) 1.4 × 10-8 m
D) 1.4 × 10-7 m
E) 2.8 × 10-8 m

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
Opposing shearing forces of 3000 N are applied to the 3.00 cm × 4.00 cm faces of a 3.00 cm × 4.00 cm × 5.00 cm steel block. By what amount does the block become slanted? The shear modulus for steel is 8.0 × 1010 N/m2.
A) 1.54 × 10-6 m
B) 5.56 × 10-7 m
C) 9.88 × 10-7 m
D) 3.08 × 10-6 m
E) 6.98 × 10-6 m
A) 1.54 × 10-6 m
B) 5.56 × 10-7 m
C) 9.88 × 10-7 m
D) 3.08 × 10-6 m
E) 6.98 × 10-6 m

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
What is the root mean square value of the following velocity components: 2.0 m/s, -3.0 m/s, and 4.0 m/s?
A) 5.4 m/s
B) 1.9 m/s
C) 3.1 m/s
D) 3.3 m/s
E) 1.0 m/s
A) 5.4 m/s
B) 1.9 m/s
C) 3.1 m/s
D) 3.3 m/s
E) 1.0 m/s

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
What is the average kinetic energy of a nitrogen molecule in the air in a room in which the air temperature is 17°C?
A) 6.01 × 10-21 J
B) 4.00 × 10-21 J
C) 5.00 × 10-21 J
D) 7.00 × 10-21 J
E) 9.00 × 10-21 J
A) 6.01 × 10-21 J
B) 4.00 × 10-21 J
C) 5.00 × 10-21 J
D) 7.00 × 10-21 J
E) 9.00 × 10-21 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
A 30-cm steel rod, 1.0 cm in diameter, supports a 300-kg mass. What is the change in length of the rod? Young's modulus for steel is 20 × 1010 N/m2.
A) 5.6 × 10-5 m
B) 6.5 × 10-5 m
C) 5.6 × 10-6 m
D) 6.5 × 10-6 m
E) 6.5 × 10-4 m
A) 5.6 × 10-5 m
B) 6.5 × 10-5 m
C) 5.6 × 10-6 m
D) 6.5 × 10-6 m
E) 6.5 × 10-4 m

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
The molecular weight of carbon dioxide is 44.0 g/mol. What is the internal energy in 3.00 kg of carbon dioxide at 300 K? As an approximation, treat the carbon dioxide gas as if it was monatomic.
A) 2.55 × 105 J
B) 1.70 × 105 J
C) 2.55 × 104 J
D) 1.70 × 104 J
E) 1.70 × 106 J
A) 2.55 × 105 J
B) 1.70 × 105 J
C) 2.55 × 104 J
D) 1.70 × 104 J
E) 1.70 × 106 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
When a mixture of gases is placed in a container at a given temperature, the pressure in the container is equal to the sum of the pressures that the individual gases would exert if they separately occupied the entire container at the same temperature. Given that air is basically a mixture of nitrogen, with a molecular weight of 28 g/mol, and oxygen, with a molecular weight of 32 g/mol, what must be the ratio of the mass of oxygen to the mass of nitrogen in air for air to have an effective molecular weight of 29?
A) 8:21
B) 3:5
C) 5:7
D) 32:87
E) 3:7
A) 8:21
B) 3:5
C) 5:7
D) 32:87
E) 3:7

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
A quantity of an ideal gas is kept in a rigid container of constant volume. If the gas is originally at a temperature of 19°C, at what temperature will the pressure of the gas double from its base value?
A) 91°C
B) 38°C
C) 311°C
D) 273°C
E) 122°C
A) 91°C
B) 38°C
C) 311°C
D) 273°C
E) 122°C

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
A 200-kg superhero, falling off a building manages to grab hold of the end of a 3.0 m-long flagpole, 5.0 cm in radius, which saves him from falling any further. After the oscillations have died down, the end of the flagpole is sagging 10 cm. What is the shear modulus of the pole?
A) 7.5 × 106 N/m2
B) 2.4 × 106 N/m2
C) 7.5 × 107 N/m2
D) 2.4 × 107 N/m2
E) 2.4 × 105 N/m2
A) 7.5 × 106 N/m2
B) 2.4 × 106 N/m2
C) 7.5 × 107 N/m2
D) 2.4 × 107 N/m2
E) 2.4 × 105 N/m2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
A 25-kg television set rests on four rubber pads, each having a height of 1.0 cm and a radius of 0.60 cm. A horizontal force is applied to the television set. When the pads are at the point of sliding, how far has the television set moved sideways? The coefficient of friction between the rubber and the floor is 1.0 and the shear modulus of rubber is 2.6 × 106 N/m2.
A) 16 mm
B) 8.4 mm
C) 4.2 mm
D) 2.1 mm
E) 1.1 mm
A) 16 mm
B) 8.4 mm
C) 4.2 mm
D) 2.1 mm
E) 1.1 mm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
What is the root mean square value of the following speeds: 2.0 m/s, 3.0 m/s, 4.0 m/s?
A) 1.0 m/s
B) 2.8 m/s
C) 2.9 m/s
D) 3.0 m/s
E) 3.1 m/s
A) 1.0 m/s
B) 2.8 m/s
C) 2.9 m/s
D) 3.0 m/s
E) 3.1 m/s

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
A 1200-kg car is being raised at a constant speed by a crane, using a 20-m long steel cable, 1.50 cm in diameter. Young's modulus for steel is 20 × 1010 N/m2. What is the change in length of the cable?
A) 6.7 mm
B) 6.7 cm
C) 3.3 mm
D) 3.3 cm
E) 3.3 m
A) 6.7 mm
B) 6.7 cm
C) 3.3 mm
D) 3.3 cm
E) 3.3 m

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
A 25-kg television set rests on four rubber pads, each having a height of 1.0 cm and a radius of 0.60 cm. A 200-N horizontal force is applied to the television set. How far does it move sideways? The shear modulus of rubber is 2.6 × 106 N/m2.
A) 6.8 mm
B) 3.4 mm
C) 1.7 mm
D) 1.5 mm
E) 1.3 mm
A) 6.8 mm
B) 3.4 mm
C) 1.7 mm
D) 1.5 mm
E) 1.3 mm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
The molecular weight of nitrogen is 28 g/mol. What is the rms speed of nitrogen molecules at 8.0°C?
A) 450 m/s
B) 500 m/s
C) 550 m/s
D) 600 m/s
E) 650 m/s
A) 450 m/s
B) 500 m/s
C) 550 m/s
D) 600 m/s
E) 650 m/s

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
A copper sphere, 10 cm in diameter, is subjected to a pressure of 1.0 × 106 N/m2. What is the change in the diameter of the sphere? The bulk modulus for copper is 1.4 × 1010 N/m2.
A) 2.2 × 10-6 m
B) 2.2 × 10-7 m
C) 7.1 × 10-6 m
D) 7.1 × 10-7 m
E) 7.1 × 10-8 m
A) 2.2 × 10-6 m
B) 2.2 × 10-7 m
C) 7.1 × 10-6 m
D) 7.1 × 10-7 m
E) 7.1 × 10-8 m

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


