Deck 18: The Laws of Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/97
Play
Full screen (f)
Deck 18: The Laws of Thermodynamics
1
The entropy of the universe remains constant during a reversible adiabatic change.
True
2
Neither heat nor work are state functions.
True
3
In the first law of thermodynamics, Q is the heat gained by the system, that is, Q is positive if the system gains heat.
True
4
On a hot day, you open the refrigerator and experience a refreshing feeling when the cool air comes in contact with your body. You think to yourself "I think that I'll just keep the refrigerator door open and stand in front of the refrigerator all day today to stay cool. I might even bring a chair." Will this work?
Explain why or why not.
Explain why or why not.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
5
State the Second Law of Thermodynamics.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
6
Internal energy is a state function.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
7
If a thermometer measures the temperature of two objects as being equal, you can conclude that the objects will be in thermal equilibrium if they are brought into thermal contact.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
8
Which has the larger entropy:
a gas that occupies only half of a container, with the other half a vacuum, or the same gas occupying the entire container?
a gas that occupies only half of a container, with the other half a vacuum, or the same gas occupying the entire container?

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
9
State the Zeroth Law of Thermodynamics.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
10
If a thermometer measures the temperature of two objects as being equal, you can conclude that if the objects are placed in thermal contact, no heat will flow between them.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
11
The entropy of the surroundings remains constant during an adiabatic change even if the change is irreversible.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
12
State the Third Law of Thermodynamics

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
13
State the First Law of Thermodynamics.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
14
The pressure falls more rapidly in a quasi-static, adiabatic process than along an isotherm when the volume of the gas is increased.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
15
All reversible engines operating between the same two temperatures have the same efficiency.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
16
Entropy is a state function.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
17
In the first law of thermodynamics, W is the work done on the system, that is, W is positive if work is done on the system.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
18
The entropy of a system remains constant during an adiabatic change even if the change is irreversible.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
19
Order in one part of the universe can only be produced at the expense of disorder in another part.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
20
A Diesel engine uses adiabatic heating to ignite the fuel.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
21
For an ideal monatomic gas,
A) Cp = Cv.
B) Cp > Cv.
C) Cp < Cv.
D) More information is needed to answer this question.
A) Cp = Cv.
B) Cp > Cv.
C) Cp < Cv.
D) More information is needed to answer this question.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
22
FIGURE 18-1 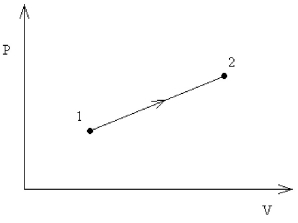
An ideal monatomic gas undergoes the reversible expansion shown in the Figure 18-1, where V2 = 5V1 and P2 = 3P1. How much heat is gained by the gas in this process, in terms of the initial pressure and volume?
A) 14 P1V1
B) 7 P1V1
C) 21 P1V1
D) 15 P1V1
E) 29 P1V1

An ideal monatomic gas undergoes the reversible expansion shown in the Figure 18-1, where V2 = 5V1 and P2 = 3P1. How much heat is gained by the gas in this process, in terms of the initial pressure and volume?
A) 14 P1V1
B) 7 P1V1
C) 21 P1V1
D) 15 P1V1
E) 29 P1V1

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
23
A monatomic ideal gas is compressed isothermically to one-third of its initial volume. The resulting pressure will be
A) three times as large as the initial value.
B) less than three times as large as the initial value.
C) more than three times as large as the initial value.
D) equal to the initial value.
E) impossible to predict on the basis of this data.
A) three times as large as the initial value.
B) less than three times as large as the initial value.
C) more than three times as large as the initial value.
D) equal to the initial value.
E) impossible to predict on the basis of this data.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
24
The molar specific heat is the amount of heat required to raise the temperature of what amount of matter by one unit of temperature?
A) one tooth
B) one unit of mass of a material
C) one mole of a substance
D) two moles of a substance
E) one molecule of a substance
A) one tooth
B) one unit of mass of a material
C) one mole of a substance
D) two moles of a substance
E) one molecule of a substance

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
25
FIGURE 18-1 
An ideal monatomic gas undergoes the reversible expansion shown in Figure 18-1, where V2 = 5V1 and P2 = 3P1. What is the change in internal energy of the gas in this process, in terms of the initial pressure and volume?
A) 7 P1V1
B) 14 P1V1
C) 21 P1V1
D) 15 P1V1
E) 29 P1V1

An ideal monatomic gas undergoes the reversible expansion shown in Figure 18-1, where V2 = 5V1 and P2 = 3P1. What is the change in internal energy of the gas in this process, in terms of the initial pressure and volume?
A) 7 P1V1
B) 14 P1V1
C) 21 P1V1
D) 15 P1V1
E) 29 P1V1

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
26
When objects at different temperatures are brought into thermal contact with one another, the resulting spontaneous flow of heat proceeds from the object with the higher
A) thermal conductivity to the one with the lower thermal conductivity.
B) specific heat to the one with the lower specific heat.
C) heat capacity to the one with the lower capacity.
D) temperature to the one with the lower temperature.
E) impossible to predict on the basis of this data.
A) thermal conductivity to the one with the lower thermal conductivity.
B) specific heat to the one with the lower specific heat.
C) heat capacity to the one with the lower capacity.
D) temperature to the one with the lower temperature.
E) impossible to predict on the basis of this data.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
27
In a given reversible process, the temperature of an ideal gas is kept constant as the gas is compressed to a smaller volume. Select the true statement from among the following:
A) The gas must absorb heat from its surroundings.
B) The gas must release heat to its surroundings.
C) The pressure of the gas also stays constant.
D) The process is adiabatic.
E) It is impossible to predict on the basis of this data.
A) The gas must absorb heat from its surroundings.
B) The gas must release heat to its surroundings.
C) The pressure of the gas also stays constant.
D) The process is adiabatic.
E) It is impossible to predict on the basis of this data.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
28
When a gas expands adiabatically,
A) the internal energy of the gas decreases.
B) the internal energy of the gas increases.
C) there is no work done by the gas.
D) work is done on the gas.
E) heat flows out of the system.
A) the internal energy of the gas decreases.
B) the internal energy of the gas increases.
C) there is no work done by the gas.
D) work is done on the gas.
E) heat flows out of the system.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
29
From the following statements regarding the ratio of the molar specific heat at constant pressure to the molar specific heat at constant volume, Cp/Cv, the only correct one for an ideal monatomic gas is
A)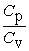 = 1.
= 1.
B)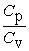 > 1.
> 1.
C)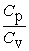 < 1.
< 1.
D) Cp/Cv is sometimes more than 1, sometimes less than 1, but never equal to 1.
E) Cp/Cv is sometimes more than 1, sometimes equal to 1, but never less than 1.
A)
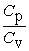 = 1.
= 1.B)
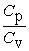 > 1.
> 1.C)
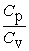 < 1.
< 1.D) Cp/Cv is sometimes more than 1, sometimes less than 1, but never equal to 1.
E) Cp/Cv is sometimes more than 1, sometimes equal to 1, but never less than 1.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
30
An ideal monatomic gas undergoes a reversible expansion to 1.5 times its original volume. In which of these processes does the gas perform the most work?
A) at constant pressure
B) if the pressure increases in proportion to the volume
C) if the pressure decreases in proportion to the volume
D) at constant temperature
E) adiabatically
A) at constant pressure
B) if the pressure increases in proportion to the volume
C) if the pressure decreases in proportion to the volume
D) at constant temperature
E) adiabatically

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
31
A monatomic ideal gas is compressed isobarically to one-third of its initial volume. The resulting pressure will be
A) three times as large as the initial value.
B) equal to the initial value.
C) more than three times as large as the initial value.
D) equal to the initial value..
E) impossible to predict on the basis of this data.
A) three times as large as the initial value.
B) equal to the initial value.
C) more than three times as large as the initial value.
D) equal to the initial value..
E) impossible to predict on the basis of this data.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
32
An ideal monatomic gas undergoes a reversible expansion to 1.5 times its original volume. In which of these processes does the gas have the largest loss of internal energy?
A) at constant pressure
B) if the pressure increases in proportion to the volume
C) if the pressure decreases in proportion to the volume
D) at constant temperature
E) adiabatically
A) at constant pressure
B) if the pressure increases in proportion to the volume
C) if the pressure decreases in proportion to the volume
D) at constant temperature
E) adiabatically

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
33
Two identical samples of a monatomic gas are initially at pressure P and occupy volume V. You perform two experiments which will both lead to the gas samples being compressed to final volumes one-third as large as their initial volumes. In one of the experiments you make the gas undergo an isothermal compression. In the second you make the gas undergo an adiabatic compression. It is correct to say that:
A) The isothermal compression leads to a higher final pressure than the adiabatic compression and the gas exchanges heat with its surroundings.
B) The adiabatic compression leads to a higher final pressure than the isothermal compression and the gas does not exchange heat with its surroundings.
C) The isothermal compression leads to a lower final pressure than the isothermal compression and the gas does not exchange heat with its surroundings.
D) The adiabatic compression leads to a lower final pressure than the isothermal compression and the gas exchanges heat with its surroundings.
E) It is impossible to predict on the basis of this data.
A) The isothermal compression leads to a higher final pressure than the adiabatic compression and the gas exchanges heat with its surroundings.
B) The adiabatic compression leads to a higher final pressure than the isothermal compression and the gas does not exchange heat with its surroundings.
C) The isothermal compression leads to a lower final pressure than the isothermal compression and the gas does not exchange heat with its surroundings.
D) The adiabatic compression leads to a lower final pressure than the isothermal compression and the gas exchanges heat with its surroundings.
E) It is impossible to predict on the basis of this data.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
34
What is meant by "the heat death of the universe"?
A) The universe will end in a giant inferno.
B) The universe will reach thermal equilibrium.
C) Some day the sun will explode and we will all burn.
D) Some day the sun will cease to provide electromagnetic radiation.
E) The radiation from the stars will continuously heat up the universe.
A) The universe will end in a giant inferno.
B) The universe will reach thermal equilibrium.
C) Some day the sun will explode and we will all burn.
D) Some day the sun will cease to provide electromagnetic radiation.
E) The radiation from the stars will continuously heat up the universe.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
35
A monatomic ideal gas is compressed adiabatically to one-third of its initial volume. The resulting pressure will be
A) three times as large as the initial value.
B) less than three times as large as the initial value.
C) more than three times as large as the initial value.
D) equal to the initial value.
E) impossible to predict on the basis of this data.
A) three times as large as the initial value.
B) less than three times as large as the initial value.
C) more than three times as large as the initial value.
D) equal to the initial value.
E) impossible to predict on the basis of this data.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
36
For water at 2°C,
A) Cp = Cv.
B) Cp > Cv.
C) Cp < Cv.
D) More information is needed to answer this question.
A) Cp = Cv.
B) Cp > Cv.
C) Cp < Cv.
D) More information is needed to answer this question.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
37
An ideal monatomic gas undergoes a reversible expansion to 1.5 times its original volume. In which of these processes does the gas perform the least amount of work?
A) at constant pressure
B) if the pressure increases in proportion to the volume
C) if the pressure decreases in proportion to the volume
D) at constant temperature
E) adiabatically
A) at constant pressure
B) if the pressure increases in proportion to the volume
C) if the pressure decreases in proportion to the volume
D) at constant temperature
E) adiabatically

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
38
If a system undergoes a reversible process,
A) it must be possible to restore the system to its original state.
B) it must be possible to restore the surroundings to their original state.
C) it must be possible to restore both the system and the surroundings to their original states.
D) it is impossible to restore either the the system or the surroundings to their original states.
E) the system must not interact with its surroundings.
A) it must be possible to restore the system to its original state.
B) it must be possible to restore the surroundings to their original state.
C) it must be possible to restore both the system and the surroundings to their original states.
D) it is impossible to restore either the the system or the surroundings to their original states.
E) the system must not interact with its surroundings.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
39
An ideal monatomic gas undergoes an isothermal expansion. It is correct to affirm that its entropy
A) decreases.
B) remains unchanged.
C) increases.
D) cannot be predicted with the data given.
A) decreases.
B) remains unchanged.
C) increases.
D) cannot be predicted with the data given.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
40
A certain gas is compressed adiabatically. The amount of work done on the gas is 800 J. What is the change in the internal energy of the gas?
A) 800 J
B) -800 J
C) 400 J
D) 0 J
E) More information is needed to answer this question.
A) 800 J
B) -800 J
C) 400 J
D) 0 J
E) More information is needed to answer this question.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
41
1.50 moles of an ideal monatomic gas are initially at a temperature of 317 K. If the gas gains 2730 J of heat and performs 780 J of work, what is its final temperature?
A) 359 K
B) 421 K
C) 526 K
D) 687 K
E) 756 K
A) 359 K
B) 421 K
C) 526 K
D) 687 K
E) 756 K

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
42
A refrigerator has a COP of 2.5. If it removes 7.7 MJ of heat in 25. minutes,
(a) what is the minimum power motor to operate the refrigerator?
(b) what is its efficiency if it were a reversible engine?
(a) what is the minimum power motor to operate the refrigerator?
(b) what is its efficiency if it were a reversible engine?

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
43
What is the change in entropy of the lead when 2.0 kg of molten lead solidifies?
[Data:
Lv = 207. kcal/kg at 1744.°C; Lf = 5.9 kcal/kg at 328.°C]
[Data:
Lv = 207. kcal/kg at 1744.°C; Lf = 5.9 kcal/kg at 328.°C]

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
44
FIGURE 18-3 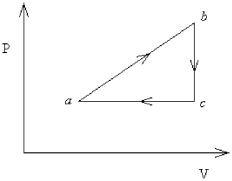
An ideal gas undergoes the process a→b→c→a shown in Figure 18-3. Pa = Pc = 240 kPa, Vb = Vc = 40 L, Va = 15 L, and Pb = 400 kPa. How much heat is gained by the system in this process?
A) 1000 J
B) 1500 J
C) 2000 J
D) 2500 J
E) 3000 J

An ideal gas undergoes the process a→b→c→a shown in Figure 18-3. Pa = Pc = 240 kPa, Vb = Vc = 40 L, Va = 15 L, and Pb = 400 kPa. How much heat is gained by the system in this process?
A) 1000 J
B) 1500 J
C) 2000 J
D) 2500 J
E) 3000 J

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
45
A heat engine absorbs 64 kcal of heat each cycle and exhausts 42 kcal.
(a) Calculate the efficiency each cycle.
(b) Calculate the work done each cycle.
(a) Calculate the efficiency each cycle.
(b) Calculate the work done each cycle.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is a statement of the third law of thermodynamics?
A) If two objects are in equilibrium with a third, then they are in thermal equilibrium with one another.
B) The entropy of the universe cannot decrease.
C) The entropy of the universe cannot increase.
D) All reversible engines operating between the same two temperatures have the same efficiency.
E) It is impossible to lower the temperature of an object to absolute zero in a finite number of steps.
A) If two objects are in equilibrium with a third, then they are in thermal equilibrium with one another.
B) The entropy of the universe cannot decrease.
C) The entropy of the universe cannot increase.
D) All reversible engines operating between the same two temperatures have the same efficiency.
E) It is impossible to lower the temperature of an object to absolute zero in a finite number of steps.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
47
FIGURE 18-3 
An ideal gas undergoes the process a→b→c→a shown in Figure 18-3. Pa = Pc = 360.0 kPa, Vb = Vc = 68.00 L, Va = 35.00 L, and Pb = 560.0 kPa. How much work is done by the system in this process?
A) 2300 J
B) 3300 J
C) 2800 J
D) 3800 J
E) 3000 J

An ideal gas undergoes the process a→b→c→a shown in Figure 18-3. Pa = Pc = 360.0 kPa, Vb = Vc = 68.00 L, Va = 35.00 L, and Pb = 560.0 kPa. How much work is done by the system in this process?
A) 2300 J
B) 3300 J
C) 2800 J
D) 3800 J
E) 3000 J

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
48
FIGURE 18-3 
An ideal gas undergoes the process a→b→c→a shown in Figure 18-3. The heat gained in process a→b is 546 J, while in process b→c the system loses 62 J. In process a→b the system performs 310 J of work, while in process c→a work is done on the system in the amount of 223 J. How much heat is gained by the system in process c→a?
A) - 397 J
B) - 62 J
C) 223 J
D) 18 J
E) - 236 J

An ideal gas undergoes the process a→b→c→a shown in Figure 18-3. The heat gained in process a→b is 546 J, while in process b→c the system loses 62 J. In process a→b the system performs 310 J of work, while in process c→a work is done on the system in the amount of 223 J. How much heat is gained by the system in process c→a?
A) - 397 J
B) - 62 J
C) 223 J
D) 18 J
E) - 236 J

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
49
One of the most efficient engines built so far has the following characteristics:
combustion chamber temperature = 1900°C; exhaust temperature = 430°C. 7.0 × 109 cal of fuel produces
1.4 × 1010 J of work in one hour. What is the power output, in hp, of this engine?
combustion chamber temperature = 1900°C; exhaust temperature = 430°C. 7.0 × 109 cal of fuel produces
1.4 × 1010 J of work in one hour. What is the power output, in hp, of this engine?

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
50
A reversible engine takes in 555. Joules per second and exhausts 482. watts.
(a) How much power does it produce?
(b) What is its efficiency?
(c) If operated in reverse as a refrigerator, what would be its COP?
(a) How much power does it produce?
(b) What is its efficiency?
(c) If operated in reverse as a refrigerator, what would be its COP?

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
51
FIGURE 18-2 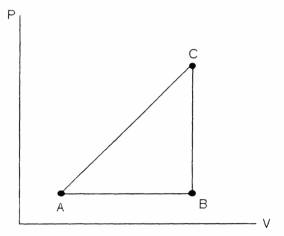
Referring to Figure 18-2, a substance carried from point A to B absorbs 50. J and finds its internal energy has increased by 20. J. Going from B to C the internal energy decreases by 5. Joules.
(a) How much work was done from A to B?
(b) How much heat was absorbed from B to C?
(c) How much work was done going from B to C?

Referring to Figure 18-2, a substance carried from point A to B absorbs 50. J and finds its internal energy has increased by 20. J. Going from B to C the internal energy decreases by 5. Joules.
(a) How much work was done from A to B?
(b) How much heat was absorbed from B to C?
(c) How much work was done going from B to C?

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
52
An engine on each cycle takes in 40. Joules, does 10. Joules of work, and expels 30. J of heat. What is its efficiency?

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
53
What is the change of entropy of water (Lf = 0.333 MJ/kg, Lv = 2.26 MJ/kg) when 450. grams of water:
(a) changes from liquid to steam?
(b) changes from ice to liquid?
(a) changes from liquid to steam?
(b) changes from ice to liquid?

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
54
One of the most efficient engines built so far has the following characteristics:
combustion chamber temperature = 1900°C, exhaust temperature = 430°C, 7.0 × 109 cal of fuel produces 1.4 × 1010 J of work in one hour.
(a) What is the actual efficiency of this engine?
(b) What is the Carnot efficiency of the engine?
(c) What is the power output, in hp, of this engine?
combustion chamber temperature = 1900°C, exhaust temperature = 430°C, 7.0 × 109 cal of fuel produces 1.4 × 1010 J of work in one hour.
(a) What is the actual efficiency of this engine?
(b) What is the Carnot efficiency of the engine?
(c) What is the power output, in hp, of this engine?

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
55
An athlete doing push-ups performs 650 kJ of work and loses 425 kJ of heat. What is the change in the internal energy of the athlete?
A) -225 kJ
B) -1075 kJ
C) 1075 kJ
D) 225 kJ
E) 276 kJ
A) -225 kJ
B) -1075 kJ
C) 1075 kJ
D) 225 kJ
E) 276 kJ

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
56
What is the change in entropy when 50. g of ice melt at 0°C?

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
57
If the efficiency of a reversible engine is 28.%,
(a) what is its COP operated as a refrigerator?
(b) what is its COP operated as a heat pump?
(a) what is its COP operated as a refrigerator?
(b) what is its COP operated as a heat pump?

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
58
An inventor tries to sell you his new engine which takes in 40. Joules of heat at 87°C on each cycle, expels 30. Joules at 27°C, and does 10. Joules of work. Why are you not fooled, and can have him prosecuted as a fraud?

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
59
A heat engine has an efficiency of 35.0% and receives 150 J of heat per cycle.
(a) How much work does it perform in each cycle?
(b) How much heat does it exhaust in each cycle?
(a) How much work does it perform in each cycle?
(b) How much heat does it exhaust in each cycle?

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
60
A gas expands from an initial volume of 30.0 L to a final volume of 65.0 L at a constant pressure of 110 kPa. How much work is done by the gas?
A) 3.85 kJ
B) 10.4 kJ
C) 3850 kJ
D) 10.4 MJ
E) 3.85 MJ
A) 3.85 kJ
B) 10.4 kJ
C) 3850 kJ
D) 10.4 MJ
E) 3.85 MJ

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
61
An expandable container holds 2.30 mole of He gas with an initial pressure of 770 kPa and an initial volume of 2.10 L. The gas expands isothermally to a final pressure of 350 kPa. How much heat is gained by the gas in this process?
A) 1280 J
B) 792 J
C) 685 J
D) 1370 J
E) 1700 J
A) 1280 J
B) 792 J
C) 685 J
D) 1370 J
E) 1700 J

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
62
A heat engine with an efficiency of 30.0% performs 2500 J of work. How much heat is discharged to the lower temperature reservoir?
A) 5830 J
B) 8330 J
C) 750 J
D) 1350 J
E) 7080 J
A) 5830 J
B) 8330 J
C) 750 J
D) 1350 J
E) 7080 J

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
63
A monatomic ideal gas with an initial pressure of 500 kPa and an initial volume of 1.80 L expands isothermally to a final volume of 5.20 L. How much work is done on the gas in this process?
A) 955 J
B) 900 J
C) 875 J
D) 1570 J
E) 1700 J
A) 955 J
B) 900 J
C) 875 J
D) 1570 J
E) 1700 J

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
64
700 J of heat are added to 12 moles of an ideal monatomic gas at constant volume. What is the change in temperature?
A) 4.7 K
B) 5.2 K
C) 5.8 J
D) 6.8 K
E) 9.3 K
A) 4.7 K
B) 5.2 K
C) 5.8 J
D) 6.8 K
E) 9.3 K

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
65
A certain ideal gas has a molar specific heat at constant volume Cv = 7R/5. What is its molar specific heat at constant pressure?
A) 12R/5
B) 7R/3
C) 12R/7
D) 12R/5.
E) 4R
A) 12R/5
B) 7R/3
C) 12R/7
D) 12R/5.
E) 4R

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
66
A monatomic ideal gas expands adiabatically from an initial volume of 72 L and an initial temperature of 350 K until its temperature falls to 290 K. What is the final volume of the gas?
A) 95 L
B) 98 L
C) 101 L
D) 104 L
E) 142 L
A) 95 L
B) 98 L
C) 101 L
D) 104 L
E) 142 L

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
67
20.0 L of a monatomic ideal gas at a pressure of 100 kPa expand adiabatically until the volume doubles. What is the pressure in the gas at that point?
A) 31.5 kPa
B) 50.0 kPa
C) 200 kPa
D) 317 kPa
E) 400 kPa
A) 31.5 kPa
B) 50.0 kPa
C) 200 kPa
D) 317 kPa
E) 400 kPa

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
68
A certain ideal gas has a value of γ = 4/3. What is its molar specific heat at constant pressure?
A) 5R/2
B) 2R
C) 3R
D) 7R/2
E) 4R
A) 5R/2
B) 2R
C) 3R
D) 7R/2
E) 4R

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
69
A monatomic ideal gas at an initial temperature of 390 K is compressed adiabatically from an initial volume of 120 L to a final volume of 40.0 L. What is the final temperature of the gas?
A) 124 K
B) 811 K
C) 610 K
D) 775 K
E) 820 K
A) 124 K
B) 811 K
C) 610 K
D) 775 K
E) 820 K

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
70
4.00 moles of a monatomic ideal gas at a temperature of 340 K are compressed adiabatically from an initial volume of 70.0 L to a final volume of 30.0 L. What is the final pressure in the gas?
A) 663 kPa
B) 581 kPa
C) 106 kPa
D) 42.1 MPa
E) 2.17 MPa
A) 663 kPa
B) 581 kPa
C) 106 kPa
D) 42.1 MPa
E) 2.17 MPa

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
71
A gas expands from an initial volume of 0.040 m3 and an initial pressure of 210 kPa to a final volume of 0.065 m3 while its temperature is kept constant. How much work is done by the system?
A) 3.7 kJ
B) 4.1 kJ
C) 5.3 kJ
D) 5.6 kJ
E) 7.9 kJ
A) 3.7 kJ
B) 4.1 kJ
C) 5.3 kJ
D) 5.6 kJ
E) 7.9 kJ

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
72
A certain engine extracts 1300 J of heat from a hot temperature reservoir and discharges 700 J of heat to a cold temperature reservoir. What is the efficiency of this engine?
A) 46%
B) 54%
C) 86%
D) 27%
E) 13%
A) 46%
B) 54%
C) 86%
D) 27%
E) 13%

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
73
2.50 moles of a monatomic ideal gas expand adiabatically from an initial temperature of 300 K and an initial volume of 80.0 L to a final volume of 150 L. What is the final temperature of the gas?
A) 840 K
B) 563 K
C) 426 K
D) 287 K
E) 197 K
A) 840 K
B) 563 K
C) 426 K
D) 287 K
E) 197 K

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
74
How much heat is required to raise the temperature of 2 moles of an ideal monatomic gas by 10 C° at constant volume?
A) 249 J
B) 416 J
C) 208 J
D) 200 J
E) 125 J
A) 249 J
B) 416 J
C) 208 J
D) 200 J
E) 125 J

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
75
How much heat is required to increase the temperature of 1.70 moles of an ideal monatomic gas by 23.0 K at constant pressure?
A) 812 J
B) 346 J
C) 751 J
D) 391 J
E) 290 J
A) 812 J
B) 346 J
C) 751 J
D) 391 J
E) 290 J

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
76
A gas expands from an initial volume of 0.040 m3 to a final volume of 0.085 m3 while its pressure increases linearly with the volume (so that the process follows a straight-line path in a P-V diagram) from 110 kPa to 225 kPa. How much work is done by the system?
A) 5.2 kJ
B) 7.5 kJ
C) 7.8 kJ
D) 11 kJ
E) 12 kJ
A) 5.2 kJ
B) 7.5 kJ
C) 7.8 kJ
D) 11 kJ
E) 12 kJ

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
77
A 40.0-L container is divided into two equal parts by a rubber membrane. One half of the container has 1.50 moles of an ideal monatomic gas at 250 K, and the other half is a vacuum. The container is well insulated, so there is no exchange of heat with the surroundings. The membrane breaks, and eventually the gas reaches a new equilibrium condition occupying the entire volume. What is the final temperature of the gas?
A) 100 K
B) 125 K
C) 157 K
D) 180 K
E) 250 K
A) 100 K
B) 125 K
C) 157 K
D) 180 K
E) 250 K

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
78
A gas expands at constant temperature from an initial volume of 0.040 m3 and an initial pressure of 210 kPa until its pressure drops to 135 kPa. How much work is done by the system?
A) 3.0 kJ
B) 3.7 kJ
C) 4.1 kJ
D) 5.6 kJ
E) 7.9 kJ
A) 3.0 kJ
B) 3.7 kJ
C) 4.1 kJ
D) 5.6 kJ
E) 7.9 kJ

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
79
3.0 moles of gas expand from an initial volume of 0.040 m3 to a final volume of 0.085 m3 while the temperature of the gas is kept fixed at 300 K. How much work is done by the system?
A) 5.6 kJ
B) 6.6 kJ
C) 7.6 kJ
D) 8.6 kJ
E) 14 kJ
A) 5.6 kJ
B) 6.6 kJ
C) 7.6 kJ
D) 8.6 kJ
E) 14 kJ

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
80
A reversible engine operating between 500 K and 300 K has the same efficiency as a reversible engine operating between 400 K and what lower temperature?
A) 200 K
B) 220 K
C) 240 K
D) 260 K
E) 280 K
A) 200 K
B) 220 K
C) 240 K
D) 260 K
E) 280 K

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck


