Exam 18: The Laws of Thermodynamics
Exam 1: Introduction to Physics100 Questions
Exam 2: One-Dimensional Kinematics112 Questions
Exam 3: Vectors in Physics82 Questions
Exam 4: Two-Dimensional Kinematics95 Questions
Exam 5: Newtons Laws of Motion101 Questions
Exam 6: Applications of Newtons Laws105 Questions
Exam 7: Work and Kinetic Energy92 Questions
Exam 8: Potential Energy and Conservation of Energy99 Questions
Exam 9: Linear Momentum and Collisions102 Questions
Exam 10: Rotational Kinematics and Energy102 Questions
Exam 11: Rotational Dynamics and Static Equilibrium97 Questions
Exam 12: Gravity94 Questions
Exam 13: Oscillations About Equilibrium102 Questions
Exam 14: Waves and Sound104 Questions
Exam 15: Fluids107 Questions
Exam 16: Temperature and Heat103 Questions
Exam 17: Phases and Phase Changes100 Questions
Exam 18: The Laws of Thermodynamics97 Questions
Exam 19: Electric Charges, Forces, and Fields88 Questions
Exam 20: Electric Potential and Electric Potential Energy99 Questions
Exam 21: Electric Current and Direct-Current Circuits99 Questions
Exam 22: Magnetism101 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction99 Questions
Exam 24: Alternating-Current Circuits93 Questions
Exam 25: Electromagnetic Waves90 Questions
Exam 26: Geometrical Optics92 Questions
Exam 27: Optical Instruments102 Questions
Exam 28: Physical Optics: Interference and Diffraction93 Questions
Exam 29: Relativity100 Questions
Exam 30: Quantum Physics100 Questions
Exam 31: Atomic Physics75 Questions
Exam 32: Nuclear Physics and Nuclear Radiation89 Questions
Select questions type
Order in one part of the universe can only be produced at the expense of disorder in another part.
Free
(True/False)
4.9/5  (31)
(31)
Correct Answer:
True
A heat engine operating at maximum efficiency has an efficiency of 35.0%. The temperature of the hot reservoir is 700 K. What is the temperature of the cold reservoir?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
D
A refrigerator has a COP of 2.5. If it removes 7.7 MJ of heat in 25. minutes,
(a) what is the minimum power motor to operate the refrigerator?
(b) what is its efficiency if it were a reversible engine?
Free
(Short Answer)
4.8/5  (26)
(26)
Correct Answer:
(a)2.1 kW
(b)29.% efficiency
The ocean thermal energy conversion project uses the surface water near tropical islands with a temperature of 20.0°C as the hot temperature reservoir, and the water at some depth, with a temperature of 5.0°C, as the cold temperature reservoir for a heat engine. What is the maximum efficiency of an engine running between those two temperatures?
(Multiple Choice)
4.8/5  (23)
(23)
An air conditioner with a coefficient of performance of 3.5 uses 30 kW of power. How much power is it discharging to the outdoors?
(Multiple Choice)
4.9/5  (36)
(36)
A gas expands from an initial volume of 30.0 L to a final volume of 65.0 L at a constant pressure of 110 kPa. How much work is done by the gas?
(Multiple Choice)
4.9/5  (29)
(29)
In a given reversible process, the temperature of an ideal gas is kept constant as the gas is compressed to a smaller volume. Select the true statement from among the following:
(Multiple Choice)
4.9/5  (37)
(37)
FIGURE 18-3 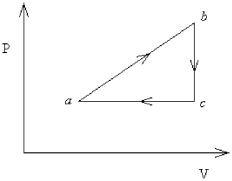 -An ideal gas undergoes the process a→b→c→a shown in Figure 18-3. Pa = Pc = 360.0 kPa, Vb = Vc = 68.00 L, Va = 35.00 L, and Pb = 560.0 kPa. How much work is done by the system in this process?
-An ideal gas undergoes the process a→b→c→a shown in Figure 18-3. Pa = Pc = 360.0 kPa, Vb = Vc = 68.00 L, Va = 35.00 L, and Pb = 560.0 kPa. How much work is done by the system in this process?
(Multiple Choice)
4.8/5  (36)
(36)
If a thermometer measures the temperature of two objects as being equal, you can conclude that the objects will be in thermal equilibrium if they are brought into thermal contact.
(True/False)
4.9/5  (42)
(42)
On a cold winter day, the outside temperature is -20°C and the inside temperature is maintained at 20°C. There is a net heat flow to the outside through the walls, roof, etc., of 25 kW. What is the rate of increase of the entropy of the universe as a result of this?
(Multiple Choice)
4.8/5  (30)
(30)
A Carnot engine has an efficiency of 83.0% and performs 4500 J of work every cycle. How much energy is discharged to the lower temperature reservoir every cycle?
(Multiple Choice)
4.9/5  (30)
(30)
An ideal monatomic gas undergoes a reversible expansion to 1.5 times its original volume. In which of these processes does the gas have the largest loss of internal energy?
(Multiple Choice)
4.9/5  (36)
(36)
FIGURE 18-2 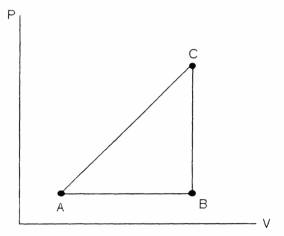 -Referring to Figure 18-2, a substance carried from point A to B absorbs 50. J and finds its internal energy has increased by 20. J. Going from B to C the internal energy decreases by 5. Joules.
(a) How much work was done from A to B?
(b) How much heat was absorbed from B to C?
(c) How much work was done going from B to C?
-Referring to Figure 18-2, a substance carried from point A to B absorbs 50. J and finds its internal energy has increased by 20. J. Going from B to C the internal energy decreases by 5. Joules.
(a) How much work was done from A to B?
(b) How much heat was absorbed from B to C?
(c) How much work was done going from B to C?
(Short Answer)
4.9/5  (25)
(25)
What is the change of entropy of water (Lf = 0.333 MJ/kg, Lv = 2.26 MJ/kg) when 450. grams of water:
(a) changes from liquid to steam?
(b) changes from ice to liquid?
(Short Answer)
4.9/5  (32)
(32)
One of the most efficient engines built so far has the following characteristics:
combustion chamber temperature = 1900°C; exhaust temperature = 430°C. 7.0 × 109 cal of fuel produces
1.4 × 1010 J of work in one hour. What is the power output, in hp, of this engine?
(Short Answer)
4.9/5  (40)
(40)
In the first law of thermodynamics, W is the work done on the system, that is, W is positive if work is done on the system.
(True/False)
4.7/5  (36)
(36)
A certain engine extracts 1300 J of heat from a hot temperature reservoir and discharges 700 J of heat to a cold temperature reservoir. What is the efficiency of this engine?
(Multiple Choice)
4.8/5  (26)
(26)
How much heat is required to raise the temperature of 2 moles of an ideal monatomic gas by 10 C° at constant volume?
(Multiple Choice)
4.8/5  (28)
(28)
On a cold winter day, the outside temperature is -20°C and the inside temperature is maintained at 20°C. There is a net heat flow to the outside through the walls, roof, etc., of 25 kW. At what rate is the air outside the house gaining entropy as a result of this process?
(Multiple Choice)
4.9/5  (32)
(32)
Showing 1 - 20 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)