Deck 6: Ionic and Molecular Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/105
Play
Full screen (f)
Deck 6: Ionic and Molecular Compounds
1
How many electrons will aluminum gain or lose when it forms an ion?
A)lose 1
B)gain 5
C)lose 2
D)lose 3
E)gain 1
A)lose 1
B)gain 5
C)lose 2
D)lose 3
E)gain 1
lose 3
2
What is the ionic charge of an ion with 13 protons and 10 electrons?
A)1+
B)2+
C)3+
D)2-
E)3-
A)1+
B)2+
C)3+
D)2-
E)3-
3+
3
Which one of the following compounds contains an ion with a 3+ charge?
A)KCl
B)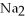 O
O
C)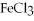
D)CuCl
E)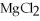
A)KCl
B)
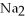 O
OC)
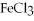
D)CuCl
E)
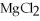
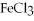
4
An anion always ________.
A)has a positive charge
B)contains a group of two or more atoms with a positive charge
C)contains a metal and a nonmetal
D)forms covalent bonds
E)has a negative charge
A)has a positive charge
B)contains a group of two or more atoms with a positive charge
C)contains a metal and a nonmetal
D)forms covalent bonds
E)has a negative charge

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
5
The compound 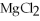 is named ________.
is named ________.
A)magnesium chlorine
B)magnesium dichloride
C)magnesium(II)chloride
D)magnesium chloride
E)dimagnesium chloride
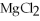 is named ________.
is named ________.A)magnesium chlorine
B)magnesium dichloride
C)magnesium(II)chloride
D)magnesium chloride
E)dimagnesium chloride

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
6
In ionic compounds,________ lose their valence electrons to form positively charged ________.
A)metals,anions
B)nonmetals,cations
C)metals,polyatomic ions
D)nonmetals,anions
E)metals,cations
A)metals,anions
B)nonmetals,cations
C)metals,polyatomic ions
D)nonmetals,anions
E)metals,cations

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
7
The correct formula for the compound formed from Mg and S is ________.
A)MgS
B)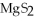
C)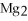 S
S
D)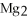
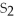
E)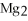
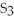
A)MgS
B)
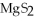
C)
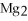 S
SD)
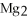
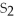
E)
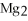
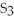

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
8
The ion of aluminum is ________.
A)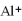
B)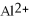
C)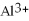
D)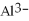
E)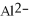
A)
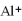
B)
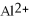
C)
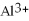
D)
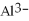
E)
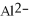

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
9
What is the symbol for the ion with 19 protons and 18 electrons?
A)F+
B)F-
C)Ar+
D)K-
E)K+
A)F+
B)F-
C)Ar+
D)K-
E)K+

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
10
What is the correct formula for the oxide ion?
A)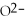
B)O
C)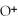
D)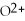
E)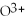
A)
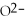
B)O
C)
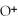
D)
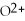
E)
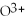

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
11
The name of the 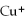 ion is ________.
ion is ________.
A)copper(II)
B)copper(I)
C)copper(III)
D)copper
E)cuprum
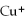 ion is ________.
ion is ________.A)copper(II)
B)copper(I)
C)copper(III)
D)copper
E)cuprum

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
12
An ionic compound ________.
A)has a net positive charge
B)has a net negative charge
C)contains only cations
D)contains only anions
E)has a net charge of zero
A)has a net positive charge
B)has a net negative charge
C)contains only cations
D)contains only anions
E)has a net charge of zero

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
13
The number of electrons in an ion with 16 protons and an ionic charge of 2- is ________.
A)16
B)18
C)20
D)22
E)24
A)16
B)18
C)20
D)22
E)24

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
14
When a cation is formed from a representative element ________.
A)electrons are gained and the ion is larger
B)electrons are gained and the ion is smaller
C)electrons are lost and the ion is larger
D)electrons are lost and the ion is smaller
E)the cation acquires a negative charge
A)electrons are gained and the ion is larger
B)electrons are gained and the ion is smaller
C)electrons are lost and the ion is larger
D)electrons are lost and the ion is smaller
E)the cation acquires a negative charge

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
15
What is the correct formula for the iron(II)ion?
A)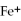
B)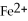
C)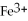
D)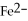
E)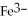
A)
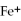
B)
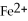
C)
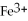
D)
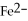
E)
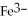

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
16
Elements in group 2A (2)of the periodic table form ions with a charge of ________.
A)1+
B)1-
C)2+
D)3+
E)0
A)1+
B)1-
C)2+
D)3+
E)0

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
17
To form an ion,a sodium atom ________.
A)gains one electron
B)gains two electrons
C)loses seven electrons
D)loses one electron
E)loses two electrons
A)gains one electron
B)gains two electrons
C)loses seven electrons
D)loses one electron
E)loses two electrons

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
18
The correct formula for a compound formed from the elements Al and O is ________.
A)AlO
B)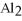 O
O
C)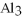
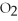
D)Al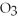
E)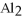
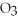
A)AlO
B)
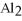 O
OC)
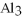
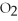
D)Al
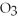
E)
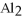
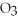

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following elements forms two or more ions with different ionic charges?
A)K
B)F
C)Ca
D)O
E)Fe
A)K
B)F
C)Ca
D)O
E)Fe

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
20
How many electrons will chlorine gain or lose when it forms an ion?
A)lose 1
B)gain 1
C)lose 7
D)gain 2
E)lose 3
A)lose 1
B)gain 1
C)lose 7
D)gain 2
E)lose 3

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
21
A(n)________ is the smallest neutral unit of two or more atoms held together by a covalent bond.
A)ionic compound
B)nucleus
C)molecule
D)formula
E)unit
A)ionic compound
B)nucleus
C)molecule
D)formula
E)unit

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
22
The name of 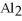
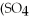
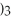 is ________.
is ________.
A)aluminum(III)sulfate
B)dialuminum trisulfate
C)dialuminum sulfate
D)dialuminum trisulfide
E)aluminum sulfate
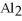
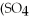
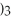 is ________.
is ________.A)aluminum(III)sulfate
B)dialuminum trisulfate
C)dialuminum sulfate
D)dialuminum trisulfide
E)aluminum sulfate

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
23
Double and triple bonds form because ________.
A)the atoms involved have high electronegativities
B)single covalent bonds do not give all of the atoms in the molecule eight valence electrons
C)one of the atoms in the molecule has more than 8 valence electrons
D)the ions involved have charges larger than one
E)there is at least one hydrogen atom involved in the bond
A)the atoms involved have high electronegativities
B)single covalent bonds do not give all of the atoms in the molecule eight valence electrons
C)one of the atoms in the molecule has more than 8 valence electrons
D)the ions involved have charges larger than one
E)there is at least one hydrogen atom involved in the bond

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following polyatomic ions has a 3- ionic charge?
A)hydroxide
B)nitrate
C)sulfate
D)phosphate
E)hydrogen carbonate
A)hydroxide
B)nitrate
C)sulfate
D)phosphate
E)hydrogen carbonate

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following polyatomic ions has a positive charge?
A)hydroxide
B)cyanide
C)hydrogen carbonate
D)ammonium
E)nitrate
A)hydroxide
B)cyanide
C)hydrogen carbonate
D)ammonium
E)nitrate

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
26
A group of covalently bonded atoms that has an overall electrical charge is called a(n)________.
A)ionic compound
B)anion
C)polyatomic ion
D)cation
E)molecule
A)ionic compound
B)anion
C)polyatomic ion
D)cation
E)molecule

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
27
In a molecule with covalent bonding,________.
A)oppositely charged ions are held together by strong electrical attractions
B)atoms of metals form bonds to atoms of nonmetals
C)atoms of different metals form bonds
D)atoms are held together by sharing electrons
E)atoms of noble gases are held together by attractions between oppositely charged ions
A)oppositely charged ions are held together by strong electrical attractions
B)atoms of metals form bonds to atoms of nonmetals
C)atoms of different metals form bonds
D)atoms are held together by sharing electrons
E)atoms of noble gases are held together by attractions between oppositely charged ions

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
28
What is the formula for aluminum nitrate?
A)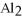
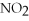
B)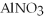
C)Al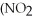
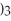
D)Al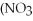
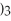
E)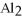
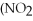
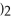
A)
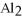
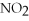
B)
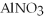
C)Al
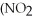
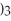
D)Al
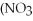
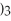
E)
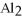
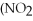
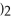

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
29
The ability of an atom to attract the shared electrons in a covalent bond is its ________.
A)electronegativity
B)bonding ability
C)polarity
D)ionic character
E)nonpolarity
A)electronegativity
B)bonding ability
C)polarity
D)ionic character
E)nonpolarity

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
30
In a covalently bonded molecule,the number of electrons that an atom shares with others is usually equal to the number of electrons ________.
A)in the atom
B)in its nucleus
C)in all the atoms
D)in its ion
E)needed to give it a noble gas arrangement
A)in the atom
B)in its nucleus
C)in all the atoms
D)in its ion
E)needed to give it a noble gas arrangement

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following elements does not exist as a diatomic molecule?
A)hydrogen
B)nitrogen
C)chlorine
D)oxygen
E)carbon
A)hydrogen
B)nitrogen
C)chlorine
D)oxygen
E)carbon

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
32
The name of the 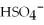 ion is ________.
ion is ________.
A)sulfate
B)hydrogen sulfate
C)sulfite
D)hydrogen sulfite
E)sulfide
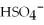 ion is ________.
ion is ________.A)sulfate
B)hydrogen sulfate
C)sulfite
D)hydrogen sulfite
E)sulfide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
33
What is the formula of carbon tetraiodide?
A)CI
B)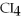
C)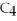 I
I
D)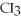
E)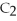
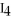
A)CI
B)
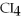
C)
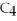 I
ID)
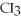
E)
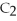
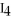

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
34
What is the formula of a compound that contains 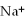 and
and 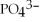 ions?
ions?
A)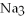
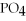
B)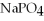
C)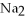
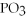
D)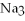

E)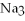 P
P
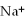 and
and 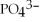 ions?
ions?A)
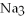
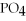
B)
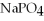
C)
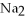
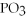
D)
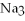

E)
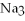 P
P
Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
35
According to naming rules,the types of compound that use prefixes in their names are ________.
A)ionic compounds
B)ionic compounds involving transition metals
C)polyatomic ions
D)molecular compounds
E)compounds that contain polyatomic ions
A)ionic compounds
B)ionic compounds involving transition metals
C)polyatomic ions
D)molecular compounds
E)compounds that contain polyatomic ions

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
36
The correct name for the compound 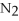
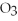 is ________.
is ________.
A)nitrogen oxide
B)nitrogen trioxide
C)dinitride trioxide
D)dinitrogen oxide
E)dinitrogen trioxide
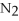
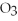 is ________.
is ________.A)nitrogen oxide
B)nitrogen trioxide
C)dinitride trioxide
D)dinitrogen oxide
E)dinitrogen trioxide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
37
The correct name of the compound 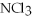 is ________.
is ________.
A)nitrogen chloride
B)trinitrogen chloride
C)nitrogen(III)chloride
D)nickel chloride
E)nitrogen trichloride
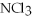 is ________.
is ________.A)nitrogen chloride
B)trinitrogen chloride
C)nitrogen(III)chloride
D)nickel chloride
E)nitrogen trichloride

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
38
What is the formula of the nitride ion?
A)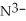
B)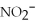
C)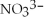
D)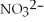
E)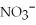
A)
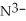
B)
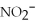
C)
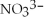
D)
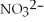
E)
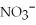

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
39
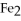
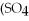
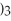 is called ________.
is called ________.A)iron sulfate
B)iron(II)sulfate
C)iron(III)sulfate
D)diiron trisulfate
E)iron trisulfate

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
40
What is the correct formula for iron(III)sulfide?
A)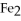
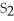
B)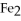 S
S
C)FeS
D)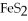
E)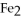
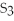
A)
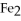
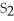
B)
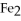 S
SC)FeS
D)
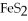
E)
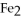
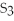

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
41
The ammonia molecule (NH3)is ________.
A)a polar molecule with polar bonds
B)a nonpolar molecule with polar bonds
C)a nonpolar molecule with nonpolar bonds
D)a polar molecule with nonpolar bonds
E)a polar molecule with ionic bonds
A)a polar molecule with polar bonds
B)a nonpolar molecule with polar bonds
C)a nonpolar molecule with nonpolar bonds
D)a polar molecule with nonpolar bonds
E)a polar molecule with ionic bonds

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
42
The carbon tetrachloride molecule,CC 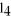 ,has the shape of a ________.
,has the shape of a ________.
A)tetrahedron
B)square
C)cube
D)circle
E)sphere
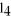 ,has the shape of a ________.
,has the shape of a ________.A)tetrahedron
B)square
C)cube
D)circle
E)sphere

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
43
The carbon tetrachloride molecule,CC 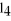 ,is ________.
,is ________.
A)a polar molecule with polar bonds
B)a nonpolar molecule with polar bonds
C)a nonpolar molecule with nonpolar bonds
D)a polar molecule with nonpolar bonds
E)a polar molecule with ionic bonds
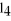 ,is ________.
,is ________.A)a polar molecule with polar bonds
B)a nonpolar molecule with polar bonds
C)a nonpolar molecule with nonpolar bonds
D)a polar molecule with nonpolar bonds
E)a polar molecule with ionic bonds

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
44
Ionic bonding is expected in which of these compounds?
A)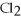
B)KF
C)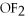
D)HF
E)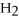
A)
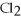
B)KF
C)
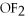
D)HF
E)
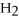

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
45
A polar covalent bond is found in which of these compounds?
A) H2O
B)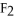
C)NaCl
D)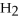
E)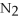
A) H2O
B)
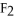
C)NaCl
D)
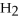
E)
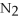

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
46
The main type of attractive forces between molecules of carbon tetrabromide (CBr4)are ________.
A)ionic bonds
B)hydrogen bonds
C)polar covalent
D)dipole-dipole attractions
E)dispersion forces
A)ionic bonds
B)hydrogen bonds
C)polar covalent
D)dipole-dipole attractions
E)dispersion forces

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
47
Identify each of the following compounds as covalent or ionic.
carbon dioxide
carbon dioxide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
48
The VSEPR theory allows us to determine the ________.
A)shape of a molecule
B)charge on an ion
C)color of a compound
D)bond type for a molecule
E)formula for a compound
A)shape of a molecule
B)charge on an ion
C)color of a compound
D)bond type for a molecule
E)formula for a compound

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
49
The shape of the water molecule (H2O)is ________.
A)linear
B)tetrahedral
C)trigonal pyramidal
D)bent
E)trigonal planar
A)linear
B)tetrahedral
C)trigonal pyramidal
D)bent
E)trigonal planar

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
50
The bond in 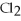 is a(n)________.
is a(n)________.
A)ionic bond
B)nonpolar covalent bond
C)metallic bond
D)polar ionic bond
E)no bond
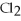 is a(n)________.
is a(n)________.A)ionic bond
B)nonpolar covalent bond
C)metallic bond
D)polar ionic bond
E)no bond

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
51
Identify each of the following compounds as covalent or ionic.
carbon tetrachloride
carbon tetrachloride

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
52
The main type of attractive forces between molecules of hydrogen (H2)are ________.
A)ionic bonds
B)hydrogen bonds
C)polar covalent
D)dipole-dipole attractions
E)dispersion forces
A)ionic bonds
B)hydrogen bonds
C)polar covalent
D)dipole-dipole attractions
E)dispersion forces

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
53
The shape of the methane molecule (CH4)is ________.
A)linear
B)tetrahedral
C)trigonal pyramidal
D)bent
E)trigonal planar
A)linear
B)tetrahedral
C)trigonal pyramidal
D)bent
E)trigonal planar

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following compounds contains an ionic bond?
A)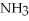
B)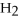 O
O
C)CaO
D)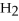
E)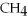
A)
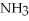
B)
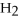 O
OC)CaO
D)
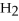
E)
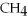

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
55
The shape of the ammonia molecule (NH3)is ________.
A)linear
B)trigonal planar
C)trigonal pyramidal
D)tetrahedral
E)bent
A)linear
B)trigonal planar
C)trigonal pyramidal
D)tetrahedral
E)bent

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following compounds contains a polar covalent bond?
A)NaF
B)HCl
C)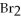
D)MgO
E)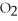
A)NaF
B)HCl
C)
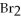
D)MgO
E)
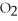

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
57
Identify each of the following compounds as covalent or ionic.
dihydrogen sulfide
dihydrogen sulfide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
58
The main type of attractive forces between molecules of ammonia (NH3)are ________.
A)ionic bonds
B)hydrogen bonds
C)polar covalent
D)dipole-dipole attractions
E)dispersion forces
A)ionic bonds
B)hydrogen bonds
C)polar covalent
D)dipole-dipole attractions
E)dispersion forces

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following substances contains a nonpolar covalent bond?
A) H2O
B)NaCl
C)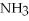
D)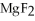
E)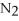
A) H2O
B)NaCl
C)
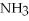
D)
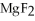
E)
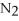

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
60
Identify each of the following compounds as covalent or ionic.
potassium oxide
potassium oxide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
61
The formula for iron(II)sulfide is FeS.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
62
The name of the compound CuSO4 is copper(I)sulfate.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
63
When barium and chlorine combine,the formula of the product is BaCl3.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
64
A sulfur atom gains electrons to form an ion with a charge of 2-.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
65
The name of the compound CuO is copper(II)oxide.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
66
The common ions of iron are Fe+ and Fe2+.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
67
Identify each of the following compounds as covalent or ionic.
sodium fluoride
sodium fluoride

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
68
When calcium and oxygen combine,the formula of the product is CaO.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
69
The formula for hydroxide is OH-.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
70
For halogens,the group number is the same as the ionic charge.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
71
The name of the compound Fe2S3 is iron(II)sulfide.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
72
A potassium atom gains electrons to form an ion with a charge of 1-.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
73
Identify each of the following compounds as covalent or ionic.
nitrogen trichloride
nitrogen trichloride

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
74
When Al3+ and SO42- combine,the formula of the product is Al2(SO4)3.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
75
When Al3+ and Br- combine,the formula of the product is Al3Br.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
76
Molecular compounds are formed from ions.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
77
The name of the compound AlCl3 is aluminum trichloride.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
78
A positive ion has more protons that electrons.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
79
The formula of the compound chromium(III)oxide is Cr2O3.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
80
The name of the compound K3N is potassium nitride.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck


