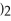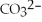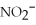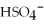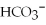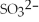Exam 6: Ionic and Molecular Compounds
Exam 1: Chemistry in Our Lives42 Questions
Exam 2: Chemistry and Measurement83 Questions
Exam 3: Matter and Energy108 Questions
Exam 4: Atoms and Elements106 Questions
Exam 5: Nuclear Chemistry89 Questions
Exam 6: Ionic and Molecular Compounds103 Questions
Exam 7: Chemical Quantities and Reactions109 Questions
Exam 8: Gases73 Questions
Exam 9: Solutions78 Questions
Exam 10: Acids and Bases and Equilibrium65 Questions
Exam 11: Introduction to Organic Chemistry: Hydrocarbons132 Questions
Exam 12: Alcohols, Thiols, Ethers, Aldehydes, and Ketones83 Questions
Exam 13: Carbohydrates82 Questions
Exam 14: Carboxylic Acids, Esters, Amines, and Amides105 Questions
Exam 15: Lipids63 Questions
Exam 16: Amino Acids, Proteins, and Enzymes107 Questions
Exam 17: Nucleic Acids and Protein Synthesis59 Questions
Exam 18: Metabolic Pathways and Energy Production136 Questions
Select questions type
A potassium atom gains electrons to form an ion with a charge of 1-.
Free
(True/False)
4.9/5  (39)
(39)
Correct Answer:
False
A nonpolar molecule can have polar bonds.
Free
(True/False)
4.9/5  (35)
(35)
Correct Answer:
True
The strongest attractive forces between molecules of Cl2 are dispersion forces.
(True/False)
4.9/5  (33)
(33)
When calcium and oxygen combine,the formula of the product is CaO.
(True/False)
4.8/5  (39)
(39)
Which of the following polyatomic ions has a positive charge?
(Multiple Choice)
4.9/5  (39)
(39)
The main type of attractive forces between molecules of carbon tetrabromide (CBr4)are ________.
(Multiple Choice)
4.8/5  (44)
(44)
Match the chemical name with the correct formula.
Correct Answer:
Premises:
Responses:
(Matching)
4.8/5  (45)
(45)
When barium and chlorine combine,the formula of the product is BaCl3.
(True/False)
4.7/5  (35)
(35)
The number of electrons in an ion with 16 protons and an ionic charge of 2- is ________.
(Multiple Choice)
4.8/5  (32)
(32)
Match the correct name of the polyatomic ions with the formulas given.
Correct Answer:
Premises:
Responses:
(Matching)
4.8/5  (29)
(29)
How many electrons will aluminum gain or lose when it forms an ion?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 103
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)