Deck 9: Molecular Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/53
Play
Full screen (f)
Deck 9: Molecular Structure
1
For which molecule will the electron-pair geometry be different than the molecular geometry?
A) NH4+
B) CO
C) CH4
D) BH3
E) H2O
A) NH4+
B) CO
C) CH4
D) BH3
E) H2O
H2O
2
Use the VSEPR model to predict the electron-pair geometry and the O-C-O bond angles for CO32-.
A) tetrahedral, 90
B) triangular planar, 120
C) bent, 120
D) linear, 180
E) bent, 180
A) tetrahedral, 90
B) triangular planar, 120
C) bent, 120
D) linear, 180
E) bent, 180
triangular planar, 120
3
The VSEPR model attempts to ____ electron-pair ____.
A) eliminate; repulsions
B) minimize; collisions
C) minimize; repulsions
D) maximize; attractions
E) maximize; repulsions
A) eliminate; repulsions
B) minimize; collisions
C) minimize; repulsions
D) maximize; attractions
E) maximize; repulsions
minimize; repulsions
4
For which molecule will the electron-pair geometry be the same as the molecular geometry?
A) NO2-
B) SeH2
C) NF3
D) ClF3
E) ICl6+
A) NO2-
B) SeH2
C) NF3
D) ClF3
E) ICl6+

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
5
According to the VSEPR model
A) The size of the electrons in the valence shell of a molecule determines the shape of the molecule.
B) The velocity of the electrons in the valence shell of a molecule determines the shape of the molecule.
C) The repulsion of electrons in the valence shell of a molecule determines the shape of the molecule.
D) The attraction of electrons in the valence shell of a molecule determines the shape of the molecule.
E) The mass of the electrons in the valence shell of a molecule determines the shape of the molecule.
A) The size of the electrons in the valence shell of a molecule determines the shape of the molecule.
B) The velocity of the electrons in the valence shell of a molecule determines the shape of the molecule.
C) The repulsion of electrons in the valence shell of a molecule determines the shape of the molecule.
D) The attraction of electrons in the valence shell of a molecule determines the shape of the molecule.
E) The mass of the electrons in the valence shell of a molecule determines the shape of the molecule.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
6
Use the VSEPR model to predict the electron-pair geometry and bond angles of PF4+.
A) octahedral, 90
B) square planar, 90
C) tetrahedral, 109.5
D) triangular pyramidal, 90
E) tetrahedral, 90
A) octahedral, 90
B) square planar, 90
C) tetrahedral, 109.5
D) triangular pyramidal, 90
E) tetrahedral, 90

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
7
Which does not provide visual insight into the three-dimensional structure of a molecule?
A) VSEPR model
B) ball-and-stick model
C) space-filling model
D) computer-generated model
E) molecular orbital model
A) VSEPR model
B) ball-and-stick model
C) space-filling model
D) computer-generated model
E) molecular orbital model

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
8
Use the VSEPR model to predict the electron-pair geometry of O3.
A) linear
B) tetrahedral
C) triangular bipyramidal
D) bent
E) triangular planar
A) linear
B) tetrahedral
C) triangular bipyramidal
D) bent
E) triangular planar

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
9
Which statement about a central atom that has six electron pairs is false?
A) The central must have an expanded octet.
B) The central atom can be a nonmetal.
C) The central atom must be from the third or subsequent periods.
D) The central atom can be a transition metal.
E) The central atom must have an octahedral molecular geometry.
A) The central must have an expanded octet.
B) The central atom can be a nonmetal.
C) The central atom must be from the third or subsequent periods.
D) The central atom can be a transition metal.
E) The central atom must have an octahedral molecular geometry.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
10
Use the VSEPR model to predict the geometry for two, three, four, five, and six electron pair domains, respectively.
A) linear, triangular pyramidal, tetrahedral, triangular bipyramidal, octahedral
B) linear, triangular planar, tetrahedral, square pyramidal, octahedral
C) bent, triangular pyramidal, tetrahedral, triangular pyramidal, octahedral
D) linear, triangular planar, tetrahedral, T-shaped, hexagonal
E) linear, triangular planar, tetrahedral, triangular bipyramidal, octahedral
A) linear, triangular pyramidal, tetrahedral, triangular bipyramidal, octahedral
B) linear, triangular planar, tetrahedral, square pyramidal, octahedral
C) bent, triangular pyramidal, tetrahedral, triangular pyramidal, octahedral
D) linear, triangular planar, tetrahedral, T-shaped, hexagonal
E) linear, triangular planar, tetrahedral, triangular bipyramidal, octahedral

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
11
Use the VSEPR model to predict the electron-pair geometry of IF2-.
A) linear
B) bent
C) triangular bipyramidal
D) tetrahedral
E) T-shaped
A) linear
B) bent
C) triangular bipyramidal
D) tetrahedral
E) T-shaped

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
12
Use the VSEPR model to predict the electron-pair geometry, the molecular geometry, and the smallest approximate F-Cl-F bond angle of ClF3.
A) triangular bipyramidal, T-shaped, 90
B) tetrahedral, triangular planar, 109.5
C) triangular bipyramidal, triangular pyramidal, 109.5
D) triangular bipyramidal, triangular planar, 120
E) triangular planar, triangular planar, 120
A) triangular bipyramidal, T-shaped, 90
B) tetrahedral, triangular planar, 109.5
C) triangular bipyramidal, triangular pyramidal, 109.5
D) triangular bipyramidal, triangular planar, 120
E) triangular planar, triangular planar, 120

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
13
Use the VSEPR model to predict the correct molecular shapes for ICl3 and NO2-.
A) triangular planar, linear
B) triangular pyramidal, triangular planar
C) see-saw, linear
D) T-shaped, bent
E) triangular bipyramidal, triangular planar
A) triangular planar, linear
B) triangular pyramidal, triangular planar
C) see-saw, linear
D) T-shaped, bent
E) triangular bipyramidal, triangular planar

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
14
When sketching a molecule, an atom connected by a dashed line means that
A) the atom has a negative formal charge.
B) the atom has a negative ionic charge.
C) the atom lies behind the plane of the paper.
D) the bond is longer than indicated by the drawing.
E) some atoms have been left out for clarity.
A) the atom has a negative formal charge.
B) the atom has a negative ionic charge.
C) the atom lies behind the plane of the paper.
D) the bond is longer than indicated by the drawing.
E) some atoms have been left out for clarity.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
15
Use the VSEPR model to predict the X-A-X angles for the following compounds: CO2, GeF4, BCl3.
A) 109.5 , 109.5 , 109.5
B) 109.5 , 90 , 90
C) 109.5 , 90 , 109.5
D) 180 , 109.5 , 120
E) 180 , 90 , 120
A) 109.5 , 109.5 , 109.5
B) 109.5 , 90 , 90
C) 109.5 , 90 , 109.5
D) 180 , 109.5 , 120
E) 180 , 90 , 120

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
16
In the VSEPR model, under which condition will the electron-pair geometry be the same as the molecular geometry?
A) in molecules with at least one lone pair of electrons on the central atom
B) in molecules with no lone pairs of electrons on the substituent atoms
C) in molecules with a central atom which is a nonmetal
D) in molecules with no lone pairs of electrons on the central atom
E) in molecules with more than one octet around the central atom
A) in molecules with at least one lone pair of electrons on the central atom
B) in molecules with no lone pairs of electrons on the substituent atoms
C) in molecules with a central atom which is a nonmetal
D) in molecules with no lone pairs of electrons on the central atom
E) in molecules with more than one octet around the central atom

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
17
How many lone pairs of electrons does the Lewis dot structure of H2S have around its central atom and what is the shape of the molecule?
A) 0, linear
B) 0, bent
C) 1, triangular planar
D) 2, tetrahedral
E) 2, bent
A) 0, linear
B) 0, bent
C) 1, triangular planar
D) 2, tetrahedral
E) 2, bent

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
18
Use the VSEPR model to predict the molecular geometry of XeF2.
A) linear
B) bent
C) tetrahedral
D) triangular bipyramidal
E) T-shaped
A) linear
B) bent
C) tetrahedral
D) triangular bipyramidal
E) T-shaped

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
19
Use the VSEPR model to predict the electron-pair geometry, the molecular geometry, and the H-N-H bond angle for NH3.
A) square planar, square planar, 90
B) tetrahedral, triangular pyramidal 109.5
C) tetrahedral, square planar, 109.5
D) triangular bipyramidal, tetrahedral 90
E) tetrahedral, tetrahedral, 109.5
A) square planar, square planar, 90
B) tetrahedral, triangular pyramidal 109.5
C) tetrahedral, square planar, 109.5
D) triangular bipyramidal, tetrahedral 90
E) tetrahedral, tetrahedral, 109.5

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following central atoms would be expected to accommodate more than four electron pairs in some of its compounds?
A) fluorine
B) boron
C) krypton
D) carbon
E) helium
A) fluorine
B) boron
C) krypton
D) carbon
E) helium

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
21
Use the VSEPR model to predict the electron-pair geometry and the molecular geometry of CO2.
A) tetrahedral, linear
B) tetrahedral, tetrahedral
C) linear, bent
D) bent, linear
E) linear, linear
A) tetrahedral, linear
B) tetrahedral, tetrahedral
C) linear, bent
D) bent, linear
E) linear, linear

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
22
Label the hybridization at C#1, C#2, C#3, and C#4 in the molecule. 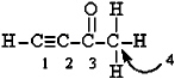 C1 C2 C3 C4
C1 C2 C3 C4
A) sp sp sp3 sp3d
B) sp sp sp2 sp3
C) sp sp2 sp2 sp2
D) sp2 sp2 sp3 sp3
E) sp3 sp3 sp3 sp3
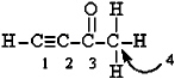 C1 C2 C3 C4
C1 C2 C3 C4A) sp sp sp3 sp3d
B) sp sp sp2 sp3
C) sp sp2 sp2 sp2
D) sp2 sp2 sp3 sp3
E) sp3 sp3 sp3 sp3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
23
The carbon-carbon bond in ethane, CH3CH3, results from the overlap of which of the following?
A) s atomic orbitals
B) p atomic orbitals
C) sp hybrid orbitals
D) sp2 hybrid orbitals
E) sp3 hybrid orbitals
A) s atomic orbitals
B) p atomic orbitals
C) sp hybrid orbitals
D) sp2 hybrid orbitals
E) sp3 hybrid orbitals

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
24
Which molecule is polar?
A) BF3
B) H2Se
C) N2
D) GeF4
E) CO2
A) BF3
B) H2Se
C) N2
D) GeF4
E) CO2

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
25
Which best describes the nature of the Si to Si bonds in a disilyne (Si2H2)?
A) three sigma
B) three pi
C) one sigma and one pi
D) two sigma and one pi
E) one sigma and two pi
A) three sigma
B) three pi
C) one sigma and one pi
D) two sigma and one pi
E) one sigma and two pi

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
26
Which description of a pi bond is false?
A) orbital overlap above and below the bonding axis
B) sideways orbital overlap
C) orbital overlap is between parallel orbitals
D) greater orbital overlap than sigma bonds
E) electron density is not along the interatomic axis
A) orbital overlap above and below the bonding axis
B) sideways orbital overlap
C) orbital overlap is between parallel orbitals
D) greater orbital overlap than sigma bonds
E) electron density is not along the interatomic axis

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
27
Which accurately describes a molecule with all polar bonds?
A) It is always a polar molecule.
B) It is never a polar molecule.
C) It may be a polar molecule.
D) It may be an ionic molecule.
E) It cannot be a nonpolar molecule.
A) It is always a polar molecule.
B) It is never a polar molecule.
C) It may be a polar molecule.
D) It may be an ionic molecule.
E) It cannot be a nonpolar molecule.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
28
Which correctly lists the relative strengths of electron-pair repulsions?
A) bonding pair-bonding pair > lone pair-bonding pair > lone pair-lone pair
B) lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair
C) lone pair-lone pair > bonding pair-bonding pair > lone pair-bonding pair
D) lone pair-bonding pair > lone pair-lone pair > bonding pair-bonding pair
E) bonding pair-bonding pair > lone pair-lone pair > lone pair-bonding pair
A) bonding pair-bonding pair > lone pair-bonding pair > lone pair-lone pair
B) lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair
C) lone pair-lone pair > bonding pair-bonding pair > lone pair-bonding pair
D) lone pair-bonding pair > lone pair-lone pair > bonding pair-bonding pair
E) bonding pair-bonding pair > lone pair-lone pair > lone pair-bonding pair

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
29
Which compound does not have tetrahedral electron-pair geometry?
A) CCl4
B) NO2-
C) H3O+
D) PCl3
E) H2O
A) CCl4
B) NO2-
C) H3O+
D) PCl3
E) H2O

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
30
Which has the smallest bond angle?
A)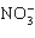
B) H2S
C) AsCl3
D) SiF4
E)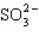
A)
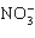
B) H2S
C) AsCl3
D) SiF4
E)
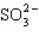

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
31
Which molecule is nonpolar?
A) NCl3
B) SO2
C) PH3
D) IF
E) CF4
A) NCl3
B) SO2
C) PH3
D) IF
E) CF4

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
32
The carbon-carbon p bond in ethylene, CH2CH2, results from the overlap of which of the following?
A) s atomic orbitals
B) p atomic orbitals
C) sp hybrid orbitals
D) sp2 hybrid orbitals
E) sp3 hybrid orbitals
A) s atomic orbitals
B) p atomic orbitals
C) sp hybrid orbitals
D) sp2 hybrid orbitals
E) sp3 hybrid orbitals

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
33
Which substances have triangular pyramidal molecular geometry? I. nitrite ion
II. hydronium ion
III. sulfite ion
IV. carbonate ion
A) I and II
B) I and III
C) II and III
D) II and IV
E) III and IV
II. hydronium ion
III. sulfite ion
IV. carbonate ion
A) I and II
B) I and III
C) II and III
D) II and IV
E) III and IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
34
If the molecular geometry of a substance is linear, the electron-pair geometry could be ____ with the central atom having ____ lone pairs.
A) triangular planar; one
B) tetrahedral; two
C) tetrahedral; four
D) triangular bipyramidal; two
E) triangular bipyramidal; three
A) triangular planar; one
B) tetrahedral; two
C) tetrahedral; four
D) triangular bipyramidal; two
E) triangular bipyramidal; three

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
35
Which statement is false? A molecule may be polar if
A) it has polar bonds
B) it has does not have nonpolar bonds
C) it is not symmetrical
D) it has a permanent dipole
E) it has all nonpolar bonds
A) it has polar bonds
B) it has does not have nonpolar bonds
C) it is not symmetrical
D) it has a permanent dipole
E) it has all nonpolar bonds

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
36
Determine the hybridization around each central atom. 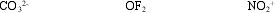
A) sp2 sp2 sp
B) sp2 sp3 sp
C) sp2 sp3 sp2
D) sp3 sp2 sp2
E) sp3 sp3 sp2
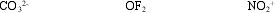
A) sp2 sp2 sp
B) sp2 sp3 sp
C) sp2 sp3 sp2
D) sp3 sp2 sp2
E) sp3 sp3 sp2

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
37
Which description of a sigma bond is false?
A) head-to-head orbital overlap
B) electron density always lies along the bonding axis
C) orbital overlap is always between hybridized orbitals
D) valence electrons are involved
E) orbital overlap is always along the interatomic axis
A) head-to-head orbital overlap
B) electron density always lies along the bonding axis
C) orbital overlap is always between hybridized orbitals
D) valence electrons are involved
E) orbital overlap is always along the interatomic axis

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
38
In a molecule with triangular bipyramidal electron-pair geometry, where are any lone pairs on the central atom preferentially placed?
A) axially
B) equatorially
C) there is no preferential placement of lone pairs
D) there can be no lone pairs on the central atom to obtain a bipyramidal electron-pair geometry
E) the placement depends on the atoms involved
A) axially
B) equatorially
C) there is no preferential placement of lone pairs
D) there can be no lone pairs on the central atom to obtain a bipyramidal electron-pair geometry
E) the placement depends on the atoms involved

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
39
How many atoms are bonded to the central atom when the molecular geometry is T-shaped?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
40
Determine the hybridization around each central atom. 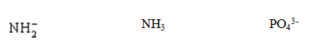
A) sp sp2 sp2
B) sp sp2 sp3
C) sp2 sp3 sp3d
D) sp2 sp3 sp3d2
E) sp3 sp3 sp3
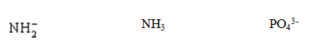
A) sp sp2 sp2
B) sp sp2 sp3
C) sp2 sp3 sp3d
D) sp2 sp3 sp3d2
E) sp3 sp3 sp3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
41
Which substance experiences dipole-dipole interactions between its molecules?
A) N2
B) ClF3
C) SiH4
D) CO2
E) PCl5
A) N2
B) ClF3
C) SiH4
D) CO2
E) PCl5

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
42
Which interaction(s) exist(s) between CO molecules? 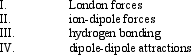
A) I only
B) II only
C) IV only
D) I and II
E) I and IV
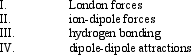
A) I only
B) II only
C) IV only
D) I and II
E) I and IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
43
Because of London forces, molecules with ____ molecular weights tend to have ____ melting points.
A) lower; higher
B) higher; lower
C) higher; higher
D) even; higher
E) odd; higher
A) lower; higher
B) higher; lower
C) higher; higher
D) even; higher
E) odd; higher

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the molecules below undergo extensive hydrogen bonding? 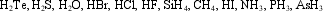
A) HBr, HCl, HF, H2O
B) H2O, HF, NH3
C) CH4, H2O, HF, NH3
D) H2S, H2O, HCl, HF
E) AsH3, NH3, HF, H2S
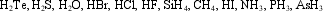
A) HBr, HCl, HF, H2O
B) H2O, HF, NH3
C) CH4, H2O, HF, NH3
D) H2S, H2O, HCl, HF
E) AsH3, NH3, HF, H2S

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
45
Which compound experiences hydrogen bonding as one of its intermolecular forces?
A) O2
B) SiH4
C) AsH3
D) HI
E) CH3OH
A) O2
B) SiH4
C) AsH3
D) HI
E) CH3OH

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
46
What is the major type of force that must be overcome to allow the boiling of carbon tetrachloride?
A) ion-dipole forces
B) dipole-dipole attractions
C) hydrogen bonding
D) covalent bonds
E) London forces
A) ion-dipole forces
B) dipole-dipole attractions
C) hydrogen bonding
D) covalent bonds
E) London forces

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
47
Which interaction(s) exist(s) between ClF3 molecules?
I. London forces.
II. ion-dipole forces
III. dipole-dipole attractions
IV. Hydrogen bonding
A) I only
B) I and III
C) II and III
D) III only
E) III and IV
I. London forces.
II. ion-dipole forces
III. dipole-dipole attractions
IV. Hydrogen bonding
A) I only
B) I and III
C) II and III
D) III only
E) III and IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
48
Characterize the polarity of these molecules, in the order written. 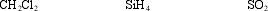
A) nonpolar, nonpolar, nonpolar
B) nonpolar, nonpolar, polar
C) polar, nonpolar, polar
D) polar, polar, nonpolar
E) polar, polar, polar
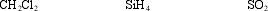
A) nonpolar, nonpolar, nonpolar
B) nonpolar, nonpolar, polar
C) polar, nonpolar, polar
D) polar, polar, nonpolar
E) polar, polar, polar

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
49
Which compound has the lowest boiling point?
A) NH3
B) CH4
C) H2O
D) HCl
E) H2Se
A) NH3
B) CH4
C) H2O
D) HCl
E) H2Se

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
50
What is the major type of force that must be overcome to allow each of the processes below? I. the evaporation of propanal (CH3CH2CH2OH)
II. the boiling of liquid CH2Cl2
III. the melting of solid Br2
IV. the boiling of liquid BF3
A) hydrogen bonding, London forces, dipole-dipole, dipole-dipole
B) hydrogen bonding, dipole-dipole, London forces, London forces
C) dipole-dipole, dipole-dipole, London forces, London forces
D) London forces, covalent bonding, dipole-dipole, dipole-dipole
E) London forces, London forces, London forces, London forces
II. the boiling of liquid CH2Cl2
III. the melting of solid Br2
IV. the boiling of liquid BF3
A) hydrogen bonding, London forces, dipole-dipole, dipole-dipole
B) hydrogen bonding, dipole-dipole, London forces, London forces
C) dipole-dipole, dipole-dipole, London forces, London forces
D) London forces, covalent bonding, dipole-dipole, dipole-dipole
E) London forces, London forces, London forces, London forces

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
51
London forces exist
A) for all molecules.
B) only for molecules with nonpolar bonds.
C) only for molecules with polar bonds.
D) only for molecules with metallic bonds.
E) only for molecules with hydrogen bonding.
A) for all molecules.
B) only for molecules with nonpolar bonds.
C) only for molecules with polar bonds.
D) only for molecules with metallic bonds.
E) only for molecules with hydrogen bonding.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
52
Samples of which substance experience only London intermolecular forces?
A) HF
B) OF2
C) PF3
D) CCl4
E) CH3Cl
A) HF
B) OF2
C) PF3
D) CCl4
E) CH3Cl

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
53
Which molecule(s) is (are) polar? 
A) I only
B) II only
C) I and II
D) I, II, and III
E) II, III, and IV

A) I only
B) II only
C) I and II
D) I, II, and III
E) II, III, and IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck


