Exam 9: Molecular Structure
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
What is the major type of force that must be overcome to allow each of the processes below? I. the evaporation of propanal (CH3CH2CH2OH)
II. the boiling of liquid CH2Cl2
III. the melting of solid Br2
IV. the boiling of liquid BF3
Free
(Multiple Choice)
4.8/5  (44)
(44)
Correct Answer:
B
Which of the molecules below undergo extensive hydrogen bonding? 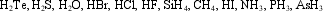
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
B
Which does not provide visual insight into the three-dimensional structure of a molecule?
(Multiple Choice)
4.7/5  (32)
(32)
Which best describes the nature of the Si to Si bonds in a disilyne (Si2H2)?
(Multiple Choice)
4.8/5  (32)
(32)
Which statement about a central atom that has six electron pairs is false?
(Multiple Choice)
4.7/5  (38)
(38)
What is the major type of force that must be overcome to allow the boiling of carbon tetrachloride?
(Multiple Choice)
4.9/5  (37)
(37)
Use the VSEPR model to predict the X-A-X angles for the following compounds: CO2, GeF4, BCl3.
(Multiple Choice)
4.7/5  (37)
(37)
Use the VSEPR model to predict the electron-pair geometry, the molecular geometry, and the smallest approximate F-Cl-F bond angle of ClF3.
(Multiple Choice)
4.8/5  (35)
(35)
Samples of which substance experience only London intermolecular forces?
(Multiple Choice)
4.9/5  (32)
(32)
Label the hybridization at C#1, C#2, C#3, and C#4 in the molecule. 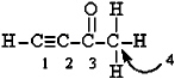 C1 C2 C3 C4
C1 C2 C3 C4
(Multiple Choice)
4.7/5  (39)
(39)
Use the VSEPR model to predict the electron-pair geometry and the molecular geometry of CO2.
(Multiple Choice)
4.9/5  (34)
(34)
Use the VSEPR model to predict the electron-pair geometry and bond angles of PF4+.
(Multiple Choice)
5.0/5  (34)
(34)
Use the VSEPR model to predict the electron-pair geometry and the O-C-O bond angles for CO32-.
(Multiple Choice)
4.8/5  (30)
(30)
The carbon-carbon bond in ethane, CH3CH3, results from the overlap of which of the following?
(Multiple Choice)
4.9/5  (29)
(29)
Which accurately describes a molecule with all polar bonds?
(Multiple Choice)
4.8/5  (27)
(27)
For which molecule will the electron-pair geometry be different than the molecular geometry?
(Multiple Choice)
4.7/5  (34)
(34)
In a molecule with triangular bipyramidal electron-pair geometry, where are any lone pairs on the central atom preferentially placed?
(Multiple Choice)
4.9/5  (31)
(31)
Showing 1 - 20 of 53
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)