Deck 8: Covalent Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 8: Covalent Bonding
1
Which statement about drawing Lewis dot structures is incorrect?
A) The element boron has three electrons in its Lewis symbol.
B) One must subtract electrons to form a cation from a neutral atom.
C) One must add electrons to form an anion from a neutral atom.
D) Not every element obeys the octet rule.
E) Second period elements can exceed the octet rule.
A) The element boron has three electrons in its Lewis symbol.
B) One must subtract electrons to form a cation from a neutral atom.
C) One must add electrons to form an anion from a neutral atom.
D) Not every element obeys the octet rule.
E) Second period elements can exceed the octet rule.
Second period elements can exceed the octet rule.
2
Which statement concerning the interaction between two atoms is incorrect?
A) If two atoms are widely separated, there is very little attraction between them.
B) When two atoms are one bond length apart, the electrons on one atom are attracted to the nucleus of the other atom.
C) When two atoms have very little separation between them, repulsion occurs.
D) A covalent bond occurs when electrons are shared between the bonded atoms.
E) As atoms get closer together, their electrons attract each other.
A) If two atoms are widely separated, there is very little attraction between them.
B) When two atoms are one bond length apart, the electrons on one atom are attracted to the nucleus of the other atom.
C) When two atoms have very little separation between them, repulsion occurs.
D) A covalent bond occurs when electrons are shared between the bonded atoms.
E) As atoms get closer together, their electrons attract each other.
As atoms get closer together, their electrons attract each other.
3
Which molecule does not contain a double bond?
A) CO2
B) CH2O
C) O2
D) HCOOH
E) HCN
A) CO2
B) CH2O
C) O2
D) HCOOH
E) HCN
HCN
4
List these compounds in order of decreasing number of valence electrons: CO2, CH3Cl, HCN.
A) CH3Cl, HCN, CO2
B) CO2, HCN, CH3Cl
C) CH3Cl, CO2, HCN
D) CO2, CH3Cl, HCN
E) HCN, CO2, CH3Cl
A) CH3Cl, HCN, CO2
B) CO2, HCN, CH3Cl
C) CH3Cl, CO2, HCN
D) CO2, CH3Cl, HCN
E) HCN, CO2, CH3Cl

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
Which statement about covalent bonds is false?
A) Covalent bonds allow atoms to achieve a filled outermost electron shell.
B) Covalent bonds are unreactive.
C) Covalent bonds occur in molecular compounds.
D) Covalent bonds involve valence electrons.
E) Covalent bonds form between non-metal atoms.
A) Covalent bonds allow atoms to achieve a filled outermost electron shell.
B) Covalent bonds are unreactive.
C) Covalent bonds occur in molecular compounds.
D) Covalent bonds involve valence electrons.
E) Covalent bonds form between non-metal atoms.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
How many electrons will be in the correctly drawn Lewis Structure for CCl4?
A) 32
B) 74
C) 35
D) 8
E) 11
A) 32
B) 74
C) 35
D) 8
E) 11

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
Which element has five electrons in its Lewis symbol?
A) argon
B) boron
C) carbon
D) phosphorus
E) sulfur
A) argon
B) boron
C) carbon
D) phosphorus
E) sulfur

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
Which element has four electrons in its Lewis symbol?
A) aluminum
B) beryllium
C) carbon
D) magnesium
E) oxygen
A) aluminum
B) beryllium
C) carbon
D) magnesium
E) oxygen

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
Which molecule contains a double bond?
A) HCN
B) H2S
C) C2H2
D) C2H6
E) O2
A) HCN
B) H2S
C) C2H2
D) C2H6
E) O2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
How many electrons will be in the correctly drawn Lewis Structure for 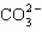 ?
?
A) 20
B) 22
C) 24
D) 26
E) 30
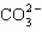 ?
?A) 20
B) 22
C) 24
D) 26
E) 30

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
Write the correct Lewis dot structure for N2. Which statement correctly describes the structure?
A) The structure contains 1 single bond 5 lone electron pairs.
B) The structure contains 1 double bond and 4 lone electron pairs.
C) The structure contains 1 double bond and 5 lone electron pairs.
D) The structure contains 1 double bond and 6 lone electron pairs.
E) The structure contains 1 triple bond and 2 lone electron pairs.
A) The structure contains 1 single bond 5 lone electron pairs.
B) The structure contains 1 double bond and 4 lone electron pairs.
C) The structure contains 1 double bond and 5 lone electron pairs.
D) The structure contains 1 double bond and 6 lone electron pairs.
E) The structure contains 1 triple bond and 2 lone electron pairs.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
Write the correct Lewis dot structure for CCl2O. Which statement correctly describes the structure?
A) The structure contains 3 single bonds, 1 double bond, and 2 lone electron pairs.
B) The structure contains 3 single bonds, 1 triple bond, and 8 lone electron pairs.
C) The structure contains 2 single bonds, 1 double bond, and 2 lone electron pairs.
D) The structure contains 2 single bonds, 1 double bond and 8 lone electron pairs.
E) The structure contains 1 single bond, 1 triple bond, and 2 lone electron pairs.
A) The structure contains 3 single bonds, 1 double bond, and 2 lone electron pairs.
B) The structure contains 3 single bonds, 1 triple bond, and 8 lone electron pairs.
C) The structure contains 2 single bonds, 1 double bond, and 2 lone electron pairs.
D) The structure contains 2 single bonds, 1 double bond and 8 lone electron pairs.
E) The structure contains 1 single bond, 1 triple bond, and 2 lone electron pairs.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
How many electrons are shown in the correctly drawn Lewis Structure for CH3CH3?
A) 8
B) 10
C) 14
D) 18
E) 34
A) 8
B) 10
C) 14
D) 18
E) 34

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
Assume all hydrocarbons given are linear. Which compound will contain one double bond?
A) C3H6
B) C6H10
C) C2H2
D) CH4
E) C5H12
A) C3H6
B) C6H10
C) C2H2
D) CH4
E) C5H12

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
Write the correct Lewis dot structure for O2. Which statement correctly describes the structure of the molecule?
A) There is one double bond and four lone electron pairs.
B) There is one double bond and six lone electron pairs.
C) There is one single bond and four lone electron pairs.
D) There is one single bond and six lone electron pairs.
E) There is one single bond, one double bond, and six lone electron pairs.
A) There is one double bond and four lone electron pairs.
B) There is one double bond and six lone electron pairs.
C) There is one single bond and four lone electron pairs.
D) There is one single bond and six lone electron pairs.
E) There is one single bond, one double bond, and six lone electron pairs.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
Which molecule does not contain a multiple bond?
A) H2O2
B) C2H2
C) CH2O
D) CO2
E) O2
A) H2O2
B) C2H2
C) CH2O
D) CO2
E) O2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
Select the element that never forms more than one bond in a Lewis dot structure.
A) O
B) C
C) N
D) Al
E) H
A) O
B) C
C) N
D) Al
E) H

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
Determine the number of electrons required by these four elements, in the order listed, to achieve an octet of electrons. 
A) 1 3 2 4
B) 3 4 2 2
C) 3 2 4 1
D) 4 3 5 2
E) 5 6 4 7

A) 1 3 2 4
B) 3 4 2 2
C) 3 2 4 1
D) 4 3 5 2
E) 5 6 4 7

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
Which molecule contains a triple bond?
A) C2H4
B) CCl4
C) H2O
D) N2
E) O2
A) C2H4
B) CCl4
C) H2O
D) N2
E) O2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
Assume all hydrocarbons given are linear. Which compound will contain one triple bond?
A) C3H6
B) C8H18
C) C4H8
D) CH4
E) C7H12
A) C3H6
B) C8H18
C) C4H8
D) CH4
E) C7H12

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
Predict the relative bond lengths of the four single bonds given below and arrange them from shortest to longest: 
A) N-C < N-Si < N-I < N-H
B) N-H < N-C < N-Si < N-I
C) N-I < N-Si < N-H < N-C
D) N-Si < N-I < N-C < NH
E) N-H < N-Si < N-C < N-I

A) N-C < N-Si < N-I < N-H
B) N-H < N-C < N-Si < N-I
C) N-I < N-Si < N-H < N-C
D) N-Si < N-I < N-C < NH
E) N-H < N-Si < N-C < N-I

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
Which statement about bond energies is false?
A) A carbon-carbon multiple bond requires less energy to break than a carbon-carbon single bond.
B) The carbon-carbon bonds in an alkane have very similar bond energies.
C) It is assumed that a carbon-hydrogen bond has the same bond energy whether it is found in an alcohol or an alkane.
D) The formation of a carbon-oxygen double bond from the unbonded atoms yields more energy than the formation of a carbon-oxygen single bond from the unbonded atoms.
E) Bond energy calculations require no knowledge of the bond polarity but they do require knowledge of the bond multiplicity and the average bond energies.
A) A carbon-carbon multiple bond requires less energy to break than a carbon-carbon single bond.
B) The carbon-carbon bonds in an alkane have very similar bond energies.
C) It is assumed that a carbon-hydrogen bond has the same bond energy whether it is found in an alcohol or an alkane.
D) The formation of a carbon-oxygen double bond from the unbonded atoms yields more energy than the formation of a carbon-oxygen single bond from the unbonded atoms.
E) Bond energy calculations require no knowledge of the bond polarity but they do require knowledge of the bond multiplicity and the average bond energies.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following compound can exhibit cis-trans isomerism?
A) CH2=CH2
B) CH3CH3
C) H2C=O
D) ClHC=CHCl
E) Cl2C=CH2
A) CH2=CH2
B) CH3CH3
C) H2C=O
D) ClHC=CHCl
E) Cl2C=CH2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
Which bond is least polar?
A) C-C
B) C-N
C) N-H
D) C-F
E) C-O
A) C-C
B) C-N
C) N-H
D) C-F
E) C-O

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
Which bond is longest?
A) C-O
B) C-P
C) C-H
D) C-C
E) C-N
A) C-O
B) C-P
C) C-H
D) C-C
E) C-N

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
Assume all hydrocarbons given are linear. Which compound will contain no multiple bonds?
A) C2H4
B) C3H4
C) C6H14
D) C8H16
E) C9H18
A) C2H4
B) C3H4
C) C6H14
D) C8H16
E) C9H18

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
Which bond is strongest?
A) carbon-nitrogen triple bond
B) carbon-nitrogen double bond
C) carbon-hydrogen single bond
D) carbon-carbon triple bond
E) carbon-carbon single bond
A) carbon-nitrogen triple bond
B) carbon-nitrogen double bond
C) carbon-hydrogen single bond
D) carbon-carbon triple bond
E) carbon-carbon single bond

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
Which represents a non-polar covalent bond?
A) H-O
B) C-N
C) C-C
D) Li-F
E) S-O
A) H-O
B) C-N
C) C-C
D) Li-F
E) S-O

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
Determine the formula for an alkyne, alkene and alkane, in this order, containing 3 carbons.
A) C3H3 C3H6 C3H4
B) C3H6 C3H8 C3H10
C) C3H4 C3H8 C3H6
D) C3H4 C3H6 C3H8
E) C3H6 C3H10 C3H8
A) C3H3 C3H6 C3H4
B) C3H6 C3H8 C3H10
C) C3H4 C3H8 C3H6
D) C3H4 C3H6 C3H8
E) C3H6 C3H10 C3H8

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
From the data below, calculate the approximate enthalpy change of reaction for the reaction below.
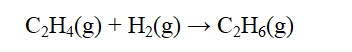

A) -392 kJ
B) -219 kJ
C) -128 kJ
D) 219 kJ
E) 392 kJ
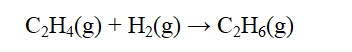

A) -392 kJ
B) -219 kJ
C) -128 kJ
D) 219 kJ
E) 392 kJ

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
Which bond is shortest?
A) carbon-oxygen double bond
B) carbon-oxygen single bond
C) carbon-nitrogen single bond
D) carbon-carbon double bond
E) carbon-nitrogen double bond
A) carbon-oxygen double bond
B) carbon-oxygen single bond
C) carbon-nitrogen single bond
D) carbon-carbon double bond
E) carbon-nitrogen double bond

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
Write the correct Lewis dot structures for the compounds given below. Arrange them in order of shortest to longest bond lengths. Consider only the carbon-carbon and carbon-oxygen bonds. 
A) C2H6 < C2H4 < C2H2 < CO
B) C2H2 < CO < C2H4 < C2H6
C) C2H2 < C2H4 < C2H6 < CO
D) CO < C2H2 < C2H4 < C2H6
E) CO < C2H6 < C2H4 < C2H2

A) C2H6 < C2H4 < C2H2 < CO
B) C2H2 < CO < C2H4 < C2H6
C) C2H2 < C2H4 < C2H6 < CO
D) CO < C2H2 < C2H4 < C2H6
E) CO < C2H6 < C2H4 < C2H2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
From the data given below, calculate the approximate enthalpy change of reaction for the reaction below.
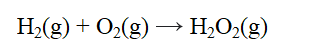

A) -490 kJ
B) -276 kJ
C) -138 kJ
D) +138 kJ
E) +325 kJ
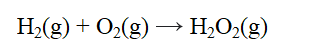

A) -490 kJ
B) -276 kJ
C) -138 kJ
D) +138 kJ
E) +325 kJ

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
In organic compounds, cis-trans isomerism is associated with which types of bonds?
A) carbon-carbon triple bonds
B) carbon-carbon single bonds
C) carbon-carbon double bonds
D) carbon-oxygen single bonds
E) carbon-oxygen double bonds
A) carbon-carbon triple bonds
B) carbon-carbon single bonds
C) carbon-carbon double bonds
D) carbon-oxygen single bonds
E) carbon-oxygen double bonds

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
Predict qualitatively the relative bond lengths of the four single bonds given below and arrange them from shortest to longest: 
A) C-C < N-N < O-O < F-F
B) N-N < O-O < F-F < C-C
C) F-F < O-O < N-N < C-C
D) O-O < N-N < F-F < C-C
E) C-C < F-F < N-N < O-O

A) C-C < N-N < O-O < F-F
B) N-N < O-O < F-F < C-C
C) F-F < O-O < N-N < C-C
D) O-O < N-N < F-F < C-C
E) C-C < F-F < N-N < O-O

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
Which bond is longest?
A) C-O
B) C-I
C) C-Br
D) C-C
E) C-N
A) C-O
B) C-I
C) C-Br
D) C-C
E) C-N

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
Which element is the most electronegative?
A) phosphorus
B) silicon
C) carbon
D) nitrogen
E) oxygen
A) phosphorus
B) silicon
C) carbon
D) nitrogen
E) oxygen

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Which statement about cis and trans isomers is false? The cis- and trans- isomers of a compound:
A) Have the same chemical formulas
B) Have the same molecular weights.
C) Occur in compounds containing triple bonds.
D) Have different boiling points.
E) Have different melting points.
A) Have the same chemical formulas
B) Have the same molecular weights.
C) Occur in compounds containing triple bonds.
D) Have different boiling points.
E) Have different melting points.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
Which bond is shortest?
A) carbon-oxygen single bond
B) carbon-hydrogen single bond
C) hydrogen-hydrogen single bond
D) carbon-carbon double bond
E) carbon-oxygen triple bond
A) carbon-oxygen single bond
B) carbon-hydrogen single bond
C) hydrogen-hydrogen single bond
D) carbon-carbon double bond
E) carbon-oxygen triple bond

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
From the data below, calculate the approximate enthalpy change for the reaction below. 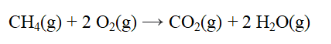

A) -806 kJ
B) -98 kJ
C) 98 kJ
D) 120 kJ
E) 806 kJ
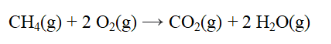

A) -806 kJ
B) -98 kJ
C) 98 kJ
D) 120 kJ
E) 806 kJ

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
Which statements about resonance are true?
I. Resonance hybrids do occur because a compound changes back and forth between two or more resonance structures.
II. Resonance structures do differ in the arrangement of electrons but not in the arrangement of atoms.
III. Resonance hybrids contain delocalized electrons.
IV. Resonance structures for a given compound always contribute equally to the resonance hybrid.
V. Resonance structures occur when there are two or more valid Lewis structures for a given compound.
VI. Resonance hybrids are a composite of resonance structures.
A) I, II, V, VI
B) II, III, IV, VI
C) II, IV, V, VI
D) II, III, V, VI
I. Resonance hybrids do occur because a compound changes back and forth between two or more resonance structures.
II. Resonance structures do differ in the arrangement of electrons but not in the arrangement of atoms.
III. Resonance hybrids contain delocalized electrons.
IV. Resonance structures for a given compound always contribute equally to the resonance hybrid.
V. Resonance structures occur when there are two or more valid Lewis structures for a given compound.
VI. Resonance hybrids are a composite of resonance structures.
A) I, II, V, VI
B) II, III, IV, VI
C) II, IV, V, VI
D) II, III, V, VI

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
Write the singly bonded Lewis dot structure for BF3. Which statement best describes this structure?
A) The octet rule is obeyed for all atoms.
B) At least one atom has less than an octet of electrons.
C) There is a lone pair of electrons on the boron atom.
D) The boron atom has a formal charge of +1.
E) At least one atom exceeds the octet rule.
A) The octet rule is obeyed for all atoms.
B) At least one atom has less than an octet of electrons.
C) There is a lone pair of electrons on the boron atom.
D) The boron atom has a formal charge of +1.
E) At least one atom exceeds the octet rule.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
What is the formal charge on sulfur in SO2?
A) +2
B) +1
C) 0
D) -1
E) -2
A) +2
B) +1
C) 0
D) -1
E) -2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
Write the correct Lewis structure for XeF4. This molecule exceeds the octet rule. Determine the number of bonding electron pairs and lone electron pairs that are present on the central atom.
A) 4 bonding pairs and 1 lone pair
B) 5 bonding pairs and 0 lone pairs
C) 4 bonding pairs and 2 lone pairs
D) 5 bonding pairs and 2 lone pairs
E) 6 bonding pairs and 0 lone pairs
A) 4 bonding pairs and 1 lone pair
B) 5 bonding pairs and 0 lone pairs
C) 4 bonding pairs and 2 lone pairs
D) 5 bonding pairs and 2 lone pairs
E) 6 bonding pairs and 0 lone pairs

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
Which of these species does not have resonance structures?
A)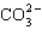
B) SO2
C) H2O
D)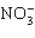
E) O3
A)
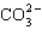
B) SO2
C) H2O
D)
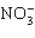
E) O3

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
Which element is the least electronegative?
A) calcium
B) cesium
C) iron
D) barium
E) potassium
A) calcium
B) cesium
C) iron
D) barium
E) potassium

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
How many resonance forms will ozone, O3, have?
A) -1
B) 0
C) 1
D) 2
E) 3
A) -1
B) 0
C) 1
D) 2
E) 3

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
Which of these species has one or more resonance structures?
A) H2S
B) CCl4
C) PCl3
D)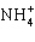
E)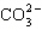
A) H2S
B) CCl4
C) PCl3
D)
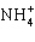
E)
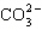

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
Construct correct Lewis dot structures for the three molecules below. Determine which compound(s) exceed the octet rule. 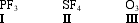
A) I
B) II
C) III
D) II and III
E) I, II, and II
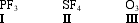
A) I
B) II
C) III
D) II and III
E) I, II, and II

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
Write the correct Lewis structure for AsCl5. This molecule exceeds the octet rule. Determine the number of bonding electron pairs and lone electron pairs that are present on the central atom.
A) 5 bonding pairs and 0 lone pairs
B) 5 bonding pairs and 1 lone pair
C) 5 bonding pairs and 2 lone pairs
D) 6 bonding pairs and 0 lone pairs
E) 6 bonding pairs and 1 lone pair
A) 5 bonding pairs and 0 lone pairs
B) 5 bonding pairs and 1 lone pair
C) 5 bonding pairs and 2 lone pairs
D) 6 bonding pairs and 0 lone pairs
E) 6 bonding pairs and 1 lone pair

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
Which of these elements cannot exceed the octet rule? 
A) Si, P, S, Cl
B) B, N, O, F
C) O, S, F, Cl
D) B, Si, N, P
E) All eight elements can exceed the octet rule.

A) Si, P, S, Cl
B) B, N, O, F
C) O, S, F, Cl
D) B, Si, N, P
E) All eight elements can exceed the octet rule.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
The correctly drawn Lewis dot structure for linear H2CO2 contains _____________ lone electron pairs, _____________ single bonds and _____________ double bonds.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
Which statement about molecular orbital theory is false?
A) Molecular orbitals are derived from valence atomic orbitals.
B) Each molecular orbital can hold two electrons.
C) Molecular orbitals hold valence electrons.
D) Molecular orbital theory is a model of covalent bonding.
E) Molecular orbitals only occur as bonding orbitals.
A) Molecular orbitals are derived from valence atomic orbitals.
B) Each molecular orbital can hold two electrons.
C) Molecular orbitals hold valence electrons.
D) Molecular orbital theory is a model of covalent bonding.
E) Molecular orbitals only occur as bonding orbitals.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Which statement properly describes the formal charges on the atoms in 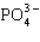 ?
?
A) -2 on oxygen, +5 on phosphorus
B) -1 on oxygen, +4 on phosphorus
C) -1 on oxygen, +1 on phosphorus
D) +1 on oxygen, -1 on phosphorus
E) +1 on oxygen, -3 on phosphorus
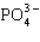 ?
?A) -2 on oxygen, +5 on phosphorus
B) -1 on oxygen, +4 on phosphorus
C) -1 on oxygen, +1 on phosphorus
D) +1 on oxygen, -1 on phosphorus
E) +1 on oxygen, -3 on phosphorus

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
What is the formal charge on carbon in CO?
A) -2
B) -1
C) 0
D) +1
E) +2
A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
Which statement properly describes the formal charges on the atoms in 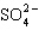 ?
?
A) +2 on sulfur, -2 on oxygen
B) +2 on sulfur, -1 on oxygen
C) +1 on sulfur, -1 on oxygen
D) -1 on sulfur, +2 on oxygen
E) -2 on sulfur, 0 on oxygen
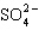 ?
?A) +2 on sulfur, -2 on oxygen
B) +2 on sulfur, -1 on oxygen
C) +1 on sulfur, -1 on oxygen
D) -1 on sulfur, +2 on oxygen
E) -2 on sulfur, 0 on oxygen

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
What is the formal charge on carbon in HCN?
A) -2
B) -1
C) 0
D) +1
E) +2
A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
Which element is the most electronegative?
A) sulfur
B) iodine
C) nitrogen
D) aluminum
E) carbon
A) sulfur
B) iodine
C) nitrogen
D) aluminum
E) carbon

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
Hydrocarbons that contain double bonds are called _____________.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
In a polar covalent bond, _____________ are _____________ shared.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
Match between columns

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
An element is allowed to have an expanded octet if empty _____________ are available.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
Oxygen atoms contribute _____________ electrons to a Lewis Dot structure.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
The ____________ of an element is its ability to pull electrons towards itself when participating in a covalent bond.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
Hydrocarbons with only single bonds are called _____________.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
The cyanide ion, CN-, has a(n) ____________ of -1 on the carbon and 0 on the nitrogen.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
Match the following:



Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
Benzene is an example of a resonance hybrid because it contains _____________ electrons.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck


