Exam 8: Covalent Bonding
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
The ____________ of an element is its ability to pull electrons towards itself when participating in a covalent bond.
Free
(Short Answer)
4.8/5  (39)
(39)
Correct Answer:
electronegativity
Write the correct Lewis dot structure for CCl2O. Which statement correctly describes the structure?
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
D
How many electrons will be in the correctly drawn Lewis Structure for CCl4?
(Multiple Choice)
4.9/5  (38)
(38)
Write the singly bonded Lewis dot structure for BF3. Which statement best describes this structure?
(Multiple Choice)
4.8/5  (31)
(31)
Write the correct Lewis dot structures for the compounds given below. Arrange them in order of shortest to longest bond lengths. Consider only the carbon-carbon and carbon-oxygen bonds. 
(Multiple Choice)
4.8/5  (32)
(32)
List these compounds in order of decreasing number of valence electrons: CO2, CH3Cl, HCN.
(Multiple Choice)
4.8/5  (33)
(33)
Which statement properly describes the formal charges on the atoms in 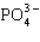 ?
?
(Multiple Choice)
4.9/5  (36)
(36)
Predict qualitatively the relative bond lengths of the four single bonds given below and arrange them from shortest to longest: 
(Multiple Choice)
4.8/5  (26)
(26)
Benzene is an example of a resonance hybrid because it contains _____________ electrons.
(Short Answer)
4.7/5  (33)
(33)
How many electrons will be in the correctly drawn Lewis Structure for 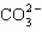 ?
?
(Multiple Choice)
4.8/5  (41)
(41)
Which statement about cis and trans isomers is false? The cis- and trans- isomers of a compound:
(Multiple Choice)
4.8/5  (33)
(33)
Assume all hydrocarbons given are linear. Which compound will contain no multiple bonds?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following compound can exhibit cis-trans isomerism?
(Multiple Choice)
4.8/5  (31)
(31)
From the data below, calculate the approximate enthalpy change for the reaction below. 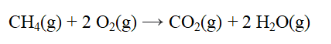

(Multiple Choice)
4.7/5  (34)
(34)
Determine the number of electrons required by these four elements, in the order listed, to achieve an octet of electrons. 
(Multiple Choice)
4.8/5  (31)
(31)
Write the correct Lewis dot structure for O2. Which statement correctly describes the structure of the molecule?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 67
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)