Deck 6: Energy and Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/56
Play
Full screen (f)
Deck 6: Energy and Chemical Reactions
1
Which substance has the highest molar heat capacity?
A) copper (specific heat = 0.385 J g-1 °C-1)
B) iron (specific heat = 0.451 J g-1 °C-1)
C) silver (specific heat = 0.232 J g-1 °C-1)
D) lead (specific heat = 0.128 J g-1 °C-1)
E) aluminum (specific heat = 0.902 J g-1 °C-1)
A) copper (specific heat = 0.385 J g-1 °C-1)
B) iron (specific heat = 0.451 J g-1 °C-1)
C) silver (specific heat = 0.232 J g-1 °C-1)
D) lead (specific heat = 0.128 J g-1 °C-1)
E) aluminum (specific heat = 0.902 J g-1 °C-1)
lead (specific heat = 0.128 J g-1 °C-1)
2
Which of the following is an example of potential energy?
A) holding a baseball
B) running around bases
C) pitching a baseball
D) swinging a bat
E) sliding into home plate
A) holding a baseball
B) running around bases
C) pitching a baseball
D) swinging a bat
E) sliding into home plate
holding a baseball
3
Which property can be used to distinguish one substance from another substance?
A) specific heat capacity
B) kinetic energy
C) temperature
D) enthalpy
E) internal energy
A) specific heat capacity
B) kinetic energy
C) temperature
D) enthalpy
E) internal energy
specific heat capacity
4
How many joules are there in one glass of milk containing 110 Calories?
A) 4.6 × 105 J
B) 460 kJ
C) 2.6 × 104 J
D) 26 J
E) 0.46 J
A) 4.6 × 105 J
B) 460 kJ
C) 2.6 × 104 J
D) 26 J
E) 0.46 J

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
5
Determine if each of the four situations below describes kinetic or potential energy.
I. the bonds in propane molecules
II. a reservoir of water behind a dam
III. water molecules colliding into each other
IV. the electrical current in a light bulb that is turned on
A)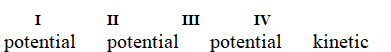
B)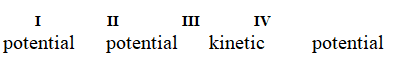
C)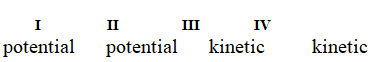
D)
E)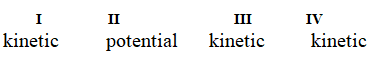
I. the bonds in propane molecules
II. a reservoir of water behind a dam
III. water molecules colliding into each other
IV. the electrical current in a light bulb that is turned on
A)
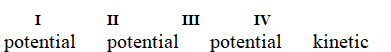
B)
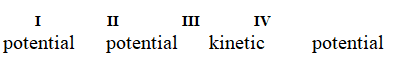
C)
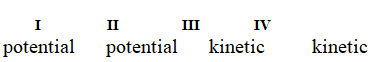
D)

E)
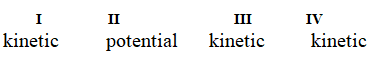

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
6
How many kilojoules are there in 150 Calories of popcorn?
A) 3.6 × 10-2 kJ
B) 36 kJ
C) 630 kJ
D) 3.6 × 104 kJ
E) 6.3 × 105 kJ
A) 3.6 × 10-2 kJ
B) 36 kJ
C) 630 kJ
D) 3.6 × 104 kJ
E) 6.3 × 105 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
7
Determine the quantity of ice required to absorb exactly 50 kJ of energy when the ice warms from -50.0°C to -10.0°C (specific heat of ice = 2.06 J g-1 °C-1).
A) 0.485 g
B) 0.607 g
C) 485 g
D) 607 g
E) 2.43 × 103 g
A) 0.485 g
B) 0.607 g
C) 485 g
D) 607 g
E) 2.43 × 103 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
8
Determine the incorrect relationship given below.
A) 1 µJ = 1 × 10-6 J
B) 1000 cal = 1 kcal
C) 44.0 kJ = 1.05 × 104 cal
D) 1000 J = 1 kJ
E) 80.0 cal/g = 312 J/g
A) 1 µJ = 1 × 10-6 J
B) 1000 cal = 1 kcal
C) 44.0 kJ = 1.05 × 104 cal
D) 1000 J = 1 kJ
E) 80.0 cal/g = 312 J/g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
9
Determine the amount of heat required to raise the temperature of a 153 g bar of gold by 50.0°C (specific heat of gold = 0.128 J g-1 °C-1).
A) 490 J
B) 979 J
C) 1.47 × 103 J
D) 7.65 × 103 J
E) 5.98 × 104 J
A) 490 J
B) 979 J
C) 1.47 × 103 J
D) 7.65 × 103 J
E) 5.98 × 104 J

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
10
The temperature of a 21.6 g sample of a metal rises 6.04°C when 58.8 J of energy is applied to it. What is the identity of the metal?
A) silver (specific heat = 0.232 J g-1 °C-1)
B) copper (specific heat = 0.385 J g-1 °C-1)
C) iron (specific heat = 0.451 J g-1 °C-1)
D) lead (specific heat = 0.128 J g-1 °C-1)
E) aluminum (specific heat = 0.902 J g-1 °C-1)
A) silver (specific heat = 0.232 J g-1 °C-1)
B) copper (specific heat = 0.385 J g-1 °C-1)
C) iron (specific heat = 0.451 J g-1 °C-1)
D) lead (specific heat = 0.128 J g-1 °C-1)
E) aluminum (specific heat = 0.902 J g-1 °C-1)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
11
What is the molar heat capacity of aluminum (specific heat = 0.902 J g-1 °C-1)?
A) 0.034 J mol-1 °C-1
B) 24.8 J mol-1 °C-1
C) 29.3 J mol-1 °C-1
D) 120 J mol-1 °C-1
E) 1.5 × 1025 J mol-1 °C-1
A) 0.034 J mol-1 °C-1
B) 24.8 J mol-1 °C-1
C) 29.3 J mol-1 °C-1
D) 120 J mol-1 °C-1
E) 1.5 × 1025 J mol-1 °C-1

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
12
The First Law of Thermodynamics states that:
A) Molecules move faster as temperature increases.
B) The total entropy of the universe is increasing.
C) Energy transfers from hotter objects to cooler objects.
D) Samples with different temperatures that come in contact with one another will reach thermal equilibrium.
E) The total energy of the universe is constant.
A) Molecules move faster as temperature increases.
B) The total entropy of the universe is increasing.
C) Energy transfers from hotter objects to cooler objects.
D) Samples with different temperatures that come in contact with one another will reach thermal equilibrium.
E) The total energy of the universe is constant.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
13
Heating a 50.0 g sample of iron (specific heat = 0.451 J g-1 °C-1) raises its temperature from 25.0°C to 79.4°C. How much energy was required to heat the sample?
A) 0.414 J
B) 2.41 J
C) 564 J
D) 1230 J
E) 1790 J
A) 0.414 J
B) 2.41 J
C) 564 J
D) 1230 J
E) 1790 J

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
14
A 20.0 g sample of aluminum (specific heat = 0.902 J g-1 °C-1) with an initial temperature of 48.6°C is heated with 427 J of energy. What is the final temperature of the sample?
A) 74.8°C
B) 72.3°C
C) 26.2°C
D) 24.9°C
E) 23.7°C
A) 74.8°C
B) 72.3°C
C) 26.2°C
D) 24.9°C
E) 23.7°C

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
15
Which statement about energy is false?
A) Thermodynamics is the science of working and heating processes.
B) Working and heating are processes that transfer energy.
C) Chemical reactions are processes that transfer energy
D) The chemical energy of foods and fuels is a type of potential energy.
E) The thermal energy of nanoscale objects is a type of potential energy.
A) Thermodynamics is the science of working and heating processes.
B) Working and heating are processes that transfer energy.
C) Chemical reactions are processes that transfer energy
D) The chemical energy of foods and fuels is a type of potential energy.
E) The thermal energy of nanoscale objects is a type of potential energy.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
16
How much energy is required to raise the temperature of 200.0 g of water by 25.0°C? The specific heat of water = 4.184 J g-1 °C-1.
A) 20.9 kJ
B) 9.36 kJ
C) 10.5 kJ
D) 210 kJ
E) 1200 kJ
A) 20.9 kJ
B) 9.36 kJ
C) 10.5 kJ
D) 210 kJ
E) 1200 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
17
Heating a 25.0 g sample of each substance below with 375 J of energy will cause which substance to have the greatest increase in temperature?
A) copper (specific heat = 0.385 J g-1 °C-1)
B) gold (specific heat = 0.128 J g-1 °C-1)
C) iron (specific heat = 0.451 J g-1 °C-1)
D) water (specific heat = 4.184 J g-1 °C-1)
E) Not enough information to determine.
A) copper (specific heat = 0.385 J g-1 °C-1)
B) gold (specific heat = 0.128 J g-1 °C-1)
C) iron (specific heat = 0.451 J g-1 °C-1)
D) water (specific heat = 4.184 J g-1 °C-1)
E) Not enough information to determine.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is not an example of kinetic energy?
A) the motion of a molecule
B) a golf ball sitting on a tee
C) the vibration of an object
D) a brick falling from the top of a building
E) the motion of electrons through a wire
A) the motion of a molecule
B) a golf ball sitting on a tee
C) the vibration of an object
D) a brick falling from the top of a building
E) the motion of electrons through a wire

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is an example of potential energy?
A) atoms vibrating back and forth around a specific location
B) a rock rolling down a mountain
C) a fat molecule stored in the body
D) electrons flowing through an electrical conductor
E) the sound of a dog barking
A) atoms vibrating back and forth around a specific location
B) a rock rolling down a mountain
C) a fat molecule stored in the body
D) electrons flowing through an electrical conductor
E) the sound of a dog barking

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
20
What is the molar heat capacity of table salt, NaCl (specific heat = 0.88 J g-1 °C-1)?
A) 5.30 × 1022 J mol-1 °C-1
B) 24.6 J mol-1 °C-1
C) 51.4 J mol-1 °C-1
D) 117 J mol-1 °C-1
E) 245 J mol-1 °C-1
A) 5.30 × 1022 J mol-1 °C-1
B) 24.6 J mol-1 °C-1
C) 51.4 J mol-1 °C-1
D) 117 J mol-1 °C-1
E) 245 J mol-1 °C-1

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
21
Based on the following thermochemical equation below, which statement is false? 
A) For the reverse process, DH° = + 46.11 kJ.
B) The value of 46.11 kJ applies to the formation of two moles of NH3.
C) The value of 92.22 kJ applies to the reaction of two moles of N2 and three moles of H2.
D) Per mole of N2, DH° = -46.11 kJ.
E) Per mole of H2, DH° = -15.37 kJ.

A) For the reverse process, DH° = + 46.11 kJ.
B) The value of 46.11 kJ applies to the formation of two moles of NH3.
C) The value of 92.22 kJ applies to the reaction of two moles of N2 and three moles of H2.
D) Per mole of N2, DH° = -46.11 kJ.
E) Per mole of H2, DH° = -15.37 kJ.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
22
The temperature of 3.50 kg of water is raised by 1.17°C when 1.00 g of hydrazine N2H4 is burned in a bomb calorimeter. The calorimeter has a heat capacity of 883 J/°C. How much heat is given off by the sample? The specific heat of water = 4.184 J g-1 °C-1.
A) 0.944 kJ
B) 16.3 kJ
C) 17.1 kJ
D) 18.2 kJ
E) 21.5 kJ
A) 0.944 kJ
B) 16.3 kJ
C) 17.1 kJ
D) 18.2 kJ
E) 21.5 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
23
Determine the heat of reaction for the process
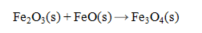
using the information given below:

A) -1074.0 kJ
B) -22.0 kJ
C) 22.2 kJ
D) 249.8 kJ
E) 1074.0 kJ
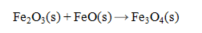
using the information given below:

A) -1074.0 kJ
B) -22.0 kJ
C) 22.2 kJ
D) 249.8 kJ
E) 1074.0 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
24
What is the enthalpy change for the combustion of 4.73 g C4H10 in excess oxygen? 
A) -23200 kJ
B) -8960 kJ
C) -401 kJ
D) -154 kJ
E) -32.7 kJ

A) -23200 kJ
B) -8960 kJ
C) -401 kJ
D) -154 kJ
E) -32.7 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
25
A 0.100 mole sample of CH4 reacts completely in a calorimeter having a heat capacity of 783 J/°C. The calorimeter contains 254 g of water. Determine the temperature increase of the calorimeter. The specific heat of water = 4.184 J g-1 °C-1. 
A) 4.35 × 10-3°C
B) 7.56°C
C) 10.2°C
D) 31.6°C
E) 43.5°C

A) 4.35 × 10-3°C
B) 7.56°C
C) 10.2°C
D) 31.6°C
E) 43.5°C

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
26
How much energy is required to melt 10.0 g of ice at 0.0°C, warm it to 100.0°C and completely vaporize the sample? The enthalpy of fusion of ice = 333 J g-1 at 0 °C; the specific heat of water = 4.184 J g-1 °C-1; the heat of vaporization of water = 2260 J g-1 at 100 °C.
A) 30100 J
B) 22600 J
C) 4180 J
D) 3330 J
E) 343 J
A) 30100 J
B) 22600 J
C) 4180 J
D) 3330 J
E) 343 J

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
27
Which process is exothermic?
A) freezing rain drops
B) evaporating alcohol
C) defrosting frozen food
D) warming milk
E) subliming dry ice
A) freezing rain drops
B) evaporating alcohol
C) defrosting frozen food
D) warming milk
E) subliming dry ice

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
28
What is the enthalpy change when 22.5 g of CH4 are burned in excess O2? 
A) -39.5 kJ
B) -890 kJ
C) -1250 kJ
D) +890 kJ
E) +1250 kJ

A) -39.5 kJ
B) -890 kJ
C) -1250 kJ
D) +890 kJ
E) +1250 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
29
A bomb calorimeter has a heat capacity of 675 J/°C and contains 925 g of water. If the combustion of 0.500 mole of a hydrocarbon increases the temperature of the calorimeter from 24.26°C to 53.88°C, determine the enthalpy change per mole of hydrocarbon. The specific heat of water = 4.184 J g-1 °C-1.
A) -94.8 kJ
B) -135 kJ
C) -229 kJ
D) -269 kJ
E) -802 kJ
A) -94.8 kJ
B) -135 kJ
C) -229 kJ
D) -269 kJ
E) -802 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
30
How much heat is required to melt 125 g of ice at 0°C? The enthalpy of fusion of ice = 333 J g-1 at 0 °C.
A) 0.375 J
B) 41.6 kJ
C) 283 kJ
D) 333 J
E) 523 J
A) 0.375 J
B) 41.6 kJ
C) 283 kJ
D) 333 J
E) 523 J

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
31
Determine if each of the four processes below describes positive or negative changes to the internal energy of the system.
I. water absorbs heat from the surroundings and becomes steam
II. steam expands and pushes against the surrounding air
III. fuel molecules burn and heat the surroundings
IV. air is compressed into an inner tube by an external pump
A)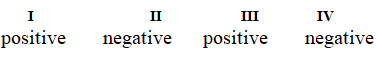
B)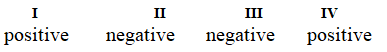
C)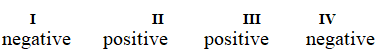
D)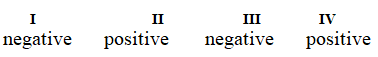
E)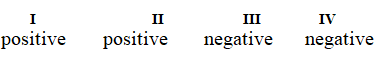
I. water absorbs heat from the surroundings and becomes steam
II. steam expands and pushes against the surrounding air
III. fuel molecules burn and heat the surroundings
IV. air is compressed into an inner tube by an external pump
A)
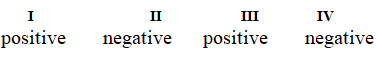
B)
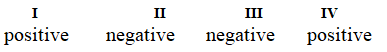
C)
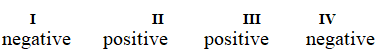
D)
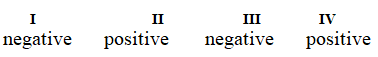
E)
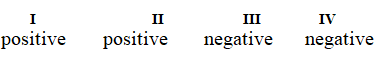

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
32
The complete combustion of 1.47 g of methanol produces 29.3 kJ of heat. Determine the DH° for the reaction and its sign. 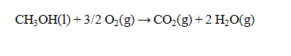
A) -938 kJ
B) -638 kJ
C) -1.35 kJ
D) +638 kJ
E) +938 kJ
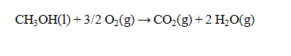
A) -938 kJ
B) -638 kJ
C) -1.35 kJ
D) +638 kJ
E) +938 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
33
When does an endothermic reaction occur?
A) when the enthalpy of the reactants is greater than the enthalpy of the products
B) when bonds are formed
C) when the energy of bonds breaking is greater than the energy of bonds formed
D) when the energy of bonds breaking is less than the energy of bonds formed
E) when stronger bonds are formed and weaker bonds are broken
A) when the enthalpy of the reactants is greater than the enthalpy of the products
B) when bonds are formed
C) when the energy of bonds breaking is greater than the energy of bonds formed
D) when the energy of bonds breaking is less than the energy of bonds formed
E) when stronger bonds are formed and weaker bonds are broken

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
34
Which term refers to a quantity of heat transferred at constant pressure?
A) work
B) specific heat capacity
C) expansion
D) entropy
E) enthalpy
A) work
B) specific heat capacity
C) expansion
D) entropy
E) enthalpy

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
35
Based on the following thermochemical equation, which statement is false? 
A) The thermochemical equation represents a physical change.
B) The enthalpy change for the gas condensing into a liquid is known.
C) The internal energy of the surroundings increases.
D) The pressure for the process is known.
E) The enthalpy change is endothermic.

A) The thermochemical equation represents a physical change.
B) The enthalpy change for the gas condensing into a liquid is known.
C) The internal energy of the surroundings increases.
D) The pressure for the process is known.
E) The enthalpy change is endothermic.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
36
A sample of water containing 2.00 moles is initially at 30.0°C. If the sample absorbs 2.00 kJ of heat, what is the final temperature of the water? (specific heat of water = 4.184 J g-1 °C-1)
A) 13.3°C
B) 30.2°C
C) 43.3°C
D) 46.7°C
E) 269°C
A) 13.3°C
B) 30.2°C
C) 43.3°C
D) 46.7°C
E) 269°C

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
37
What is the enthalpy change when 175 g of C3H8 are burned in excess O2? 
A) -1.71 × 107 kJ
B) -1.79 × 10-3 kJ
C) -3.47 × 100 kJ
D) -3.89 × 105 kJ
E) -8.82 × 103 kJ

A) -1.71 × 107 kJ
B) -1.79 × 10-3 kJ
C) -3.47 × 100 kJ
D) -3.89 × 105 kJ
E) -8.82 × 103 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
38
Determine the heat of reaction for the process
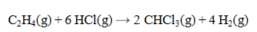
using the information given below:

A) -295.3 kJ
B) -29.2 kJ
C) +29.2 kJ
D) +295.3 kJ
E) +398.4 kJ
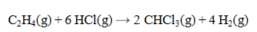
using the information given below:

A) -295.3 kJ
B) -29.2 kJ
C) +29.2 kJ
D) +295.3 kJ
E) +398.4 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following statements is false?
A) Breaking bonds is always endothermic.
B) Bond enthalpies quantify the energy change for the complete separation of two bonded atoms in a molecule at constant pressure.
C) Bond enthalpy values are based on molecules in the gas phase.
D) Breaking weak bonds and making an equal number of strong bonds is exothermic.
E) Breaking weak bonds and making a greater number of equally weak bonds is endothermic.
A) Breaking bonds is always endothermic.
B) Bond enthalpies quantify the energy change for the complete separation of two bonded atoms in a molecule at constant pressure.
C) Bond enthalpy values are based on molecules in the gas phase.
D) Breaking weak bonds and making an equal number of strong bonds is exothermic.
E) Breaking weak bonds and making a greater number of equally weak bonds is endothermic.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
40
A bomb calorimeter has a heat capacity of 783 J/°C and contains 254 g of water. How much energy is evolved or absorbed when the temperature of the calorimeter goes from 23.73°C to 26.01°C? The specific heat of water = 4.184 J g-1 °C-1.
A) 1.78 kJ evolved
B) 2.42 kJ evolved
C) 4.21 kJ evolved
D) 2420 kJ absorbed
E) 4210 kJ evolved
A) 1.78 kJ evolved
B) 2.42 kJ evolved
C) 4.21 kJ evolved
D) 2420 kJ absorbed
E) 4210 kJ evolved

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
41
Determine the heat of reaction for the process 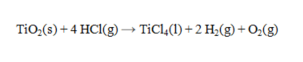
using the information given below:

A) -320.1 kJ
B) -233.7 kJ
C) 233.7 kJ
D) 320.1 kJ
E) 504.7 kJ
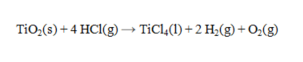
using the information given below:

A) -320.1 kJ
B) -233.7 kJ
C) 233.7 kJ
D) 320.1 kJ
E) 504.7 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
42
According to the First Law of Thermodynamics, the total _____________ of the universe is constant.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
43
The quantity of energy required to increase the temperature of one gram of a sample by 1°C is called the _____________.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
44
Match between columns

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
45
Enthalpy change is equal to heat transfer at constant _______________.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
46
The standard enthalpies of formation for several substances are given below:  Determine the DH° for the reaction below.
Determine the DH° for the reaction below.
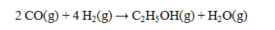
A) -366.4 kJ
B) -299.9 kJ
C) -298.5 kJ
D) -255.9 kJ
E) 255.9 kJ
 Determine the DH° for the reaction below.
Determine the DH° for the reaction below.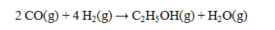
A) -366.4 kJ
B) -299.9 kJ
C) -298.5 kJ
D) -255.9 kJ
E) 255.9 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
47
How much energy in kilojoules is required to raise the temperature of 20.0 g of water from 22.0°C to 37.0°C? The specific heat of water = 4.184 J g-1 °C-1.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
48
The standard enthalpies of formation for several substances are given below:  Calculate the DH° for the reaction below.
Calculate the DH° for the reaction below.
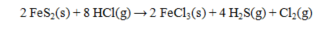
A) -881.4 kJ
B) -811.8 kJ
C) -149.6 kJ
D) +149.6 kJ
E) +213.4 kJ
 Calculate the DH° for the reaction below.
Calculate the DH° for the reaction below.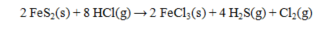
A) -881.4 kJ
B) -811.8 kJ
C) -149.6 kJ
D) +149.6 kJ
E) +213.4 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
49
The standard enthalpies of formation for several substances are given below:  Determine the DH° for the reaction below.
Determine the DH° for the reaction below.
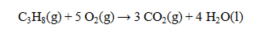
A) -2322.5 kJ
B) -2219.9 kJ
C) -782.8 kJ
D) -575.2.7 kJ
E) +575.2 kJ
 Determine the DH° for the reaction below.
Determine the DH° for the reaction below.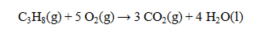
A) -2322.5 kJ
B) -2219.9 kJ
C) -782.8 kJ
D) -575.2.7 kJ
E) +575.2 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
50
In an endothermic reaction, heat is transferred from the _____________ to the _____________.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
51
The standard enthalpies of formation for several substances are given below:  Calculate the DH° for the reaction below.
Calculate the DH° for the reaction below.
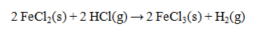
A) -219.0 kJ
B) -69.2 kJ
C) 34.6 kJ
D) 69.2 kJ
E) 219.0 kJ
 Calculate the DH° for the reaction below.
Calculate the DH° for the reaction below.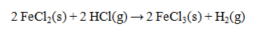
A) -219.0 kJ
B) -69.2 kJ
C) 34.6 kJ
D) 69.2 kJ
E) 219.0 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
52
Energy of motion is called _____________.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
53
The standard enthalpies of formation for several substances are given below:  Determine the heat of vaporization for H2O and C2H5OH.
Determine the heat of vaporization for H2O and C2H5OH.
A) 241.8 and -235.1 kJ
B) 241.8 and 325.1 kJ
C) 44.0 and 42.6 kJ
D) -54.0 and -42.6 kJ
E) -44.0 and -42.6 kJ
 Determine the heat of vaporization for H2O and C2H5OH.
Determine the heat of vaporization for H2O and C2H5OH.A) 241.8 and -235.1 kJ
B) 241.8 and 325.1 kJ
C) 44.0 and 42.6 kJ
D) -54.0 and -42.6 kJ
E) -44.0 and -42.6 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
54
In a(n) _____________ reaction, the energy of bond breaking is greater than the energy of bond making.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
Match the following:



Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
How much energy in kilojoules is required to vaporize a 25.0 g sample of water at 100.0°C? The heat of vaporization of water = 2260 J g-1 at 100°C.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck


