Exam 6: Energy and Chemical Reactions
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
A 0.100 mole sample of CH4 reacts completely in a calorimeter having a heat capacity of 783 J/°C. The calorimeter contains 254 g of water. Determine the temperature increase of the calorimeter. The specific heat of water = 4.184 J g-1 °C-1. 
Free
(Multiple Choice)
4.7/5  (38)
(38)
Correct Answer:
E
The quantity of energy required to increase the temperature of one gram of a sample by 1°C is called the _____________.
Free
(Short Answer)
4.9/5  (39)
(39)
Correct Answer:
specific heat
Which of the following is an example of potential energy?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
C
The temperature of a 21.6 g sample of a metal rises 6.04°C when 58.8 J of energy is applied to it. What is the identity of the metal?
(Multiple Choice)
4.8/5  (26)
(26)
Determine the heat of reaction for the process
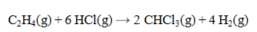 using the information given below:
using the information given below:

(Multiple Choice)
4.8/5  (39)
(39)
What is the enthalpy change when 175 g of C3H8 are burned in excess O2? 
(Multiple Choice)
4.8/5  (34)
(34)
In a(n) _____________ reaction, the energy of bond breaking is greater than the energy of bond making.
(Short Answer)
4.7/5  (30)
(30)
Determine the quantity of ice required to absorb exactly 50 kJ of energy when the ice warms from -50.0°C to -10.0°C (specific heat of ice = 2.06 J g-1 °C-1).
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following is not an example of kinetic energy?
(Multiple Choice)
4.8/5  (39)
(39)
Determine the heat of reaction for the process
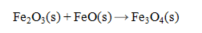 using the information given below:
using the information given below:

(Multiple Choice)
4.8/5  (35)
(35)
In an endothermic reaction, heat is transferred from the _____________ to the _____________.
(Short Answer)
4.9/5  (40)
(40)
How much energy is required to melt 10.0 g of ice at 0.0°C, warm it to 100.0°C and completely vaporize the sample? The enthalpy of fusion of ice = 333 J g-1 at 0 °C; the specific heat of water = 4.184 J g-1 °C-1; the heat of vaporization of water = 2260 J g-1 at 100 °C.
(Multiple Choice)
4.8/5  (36)
(36)
A 20.0 g sample of aluminum (specific heat = 0.902 J g-1 °C-1) with an initial temperature of 48.6°C is heated with 427 J of energy. What is the final temperature of the sample?
(Multiple Choice)
4.8/5  (31)
(31)
Based on the following thermochemical equation, which statement is false? 
(Multiple Choice)
4.9/5  (35)
(35)
Determine the amount of heat required to raise the temperature of a 153 g bar of gold by 50.0°C (specific heat of gold = 0.128 J g-1 °C-1).
(Multiple Choice)
4.8/5  (27)
(27)
The complete combustion of 1.47 g of methanol produces 29.3 kJ of heat. Determine the DH° for the reaction and its sign. 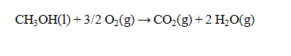
(Multiple Choice)
4.8/5  (34)
(34)
Based on the following thermochemical equation below, which statement is false? 
(Multiple Choice)
4.9/5  (28)
(28)
Showing 1 - 20 of 55
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)