Deck 5: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/67
Play
Full screen (f)
Deck 5: Chemical Reactions
1
Which statement about the reaction below is true, given large amounts of reactants? 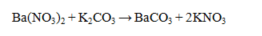
A) Ba(NO3)2 is insoluble in water and will precipitate.
B) K2CO3 is insoluble in water and will precipitate.
C) BaCO3 is insoluble in water and will precipitate.
D) Both BaCO3 and KNO3 are insoluble in water and will precipitate.
E) All compounds in the reaction are soluble in water and no reaction occurs.
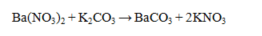
A) Ba(NO3)2 is insoluble in water and will precipitate.
B) K2CO3 is insoluble in water and will precipitate.
C) BaCO3 is insoluble in water and will precipitate.
D) Both BaCO3 and KNO3 are insoluble in water and will precipitate.
E) All compounds in the reaction are soluble in water and no reaction occurs.
BaCO3 is insoluble in water and will precipitate.
2
All of the following are strong electrolytes except:
A) CH3COOH.
B) H2SO4.
C) HNO3.
D) HBr.
E) NaOH.
A) CH3COOH.
B) H2SO4.
C) HNO3.
D) HBr.
E) NaOH.
CH3COOH.
3
Which compound will dissolve in water in large amounts?
A) AgCl
B) Al(OH)3
C) Na2SO4
D) BaSO4
E) MgS
A) AgCl
B) Al(OH)3
C) Na2SO4
D) BaSO4
E) MgS
Na2SO4
4
Which statement about bases is true?
A) Bases increase the hydroxide ion concentration of water when dissolved in it.
B) Bases increase the hydronium ion concentration of water when dissolved in it.
C) Bases turn phenolphthlalein colorless.
D) Bases react with limestone to produce gas bubbles.
E) Bases react with many metals to produce a flammable gas.
A) Bases increase the hydroxide ion concentration of water when dissolved in it.
B) Bases increase the hydronium ion concentration of water when dissolved in it.
C) Bases turn phenolphthlalein colorless.
D) Bases react with limestone to produce gas bubbles.
E) Bases react with many metals to produce a flammable gas.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is a nonelectrolyte?
A) Mg(OH)2
B) NaCl
C) KI
D) HBr
E) C6H12O6
A) Mg(OH)2
B) NaCl
C) KI
D) HBr
E) C6H12O6

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is a reducing agent?
A) I2
B) Na
C) O2
D) F2
E) Br2
A) I2
B) Na
C) O2
D) F2
E) Br2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
7
Which statement about the reaction below is true, given large amounts of reactants? 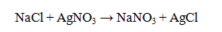
A) NaNO3 is insoluble in water and will precipitate.
B) AgCl is insoluble in water and will precipitate.
C) Both NaNO3 and AgCl are insoluble in water and will precipitate.
D) AgNO3 is insoluble in water and no reaction will occur.
E) All compounds in the reaction are soluble in water and no reaction occurs.
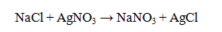
A) NaNO3 is insoluble in water and will precipitate.
B) AgCl is insoluble in water and will precipitate.
C) Both NaNO3 and AgCl are insoluble in water and will precipitate.
D) AgNO3 is insoluble in water and no reaction will occur.
E) All compounds in the reaction are soluble in water and no reaction occurs.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
8
What is the reducing agent in the reaction below? 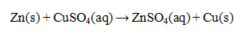
A) Zn
B) Cu2+
C) CuSO4
D) Zn2+
E) SO42-
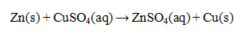
A) Zn
B) Cu2+
C) CuSO4
D) Zn2+
E) SO42-

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
9
What is the correct formula for the hydroxide ion?
A) H3O+
B) OH-
C) NH4+
D) H+
E) H-
A) H3O+
B) OH-
C) NH4+
D) H+
E) H-

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
10
What is the net ionic equation for the reaction of AlCl3 and NaOH?
A) Na+ + Cl- NaCl
B) Al+ + OH- AlOH
C) Al3+ + 3OH- Al(OH)3
D) Al3+ + OH3- AlOH
E) Na3+ + 3Cl- NaCl3
A) Na+ + Cl- NaCl
B) Al+ + OH- AlOH
C) Al3+ + 3OH- Al(OH)3
D) Al3+ + OH3- AlOH
E) Na3+ + 3Cl- NaCl3

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following best describes an acid?
A) Acids react with many metals to produce a flammable gas.
B) Acids react with many metals to produce precipitates.
C) Acids increase the hydrogen ion concentration of water when dissolved in it.
D) Both a and c.
E) All of these.
A) Acids react with many metals to produce a flammable gas.
B) Acids react with many metals to produce precipitates.
C) Acids increase the hydrogen ion concentration of water when dissolved in it.
D) Both a and c.
E) All of these.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
12
Which one or two substances are reduced in the reaction below? 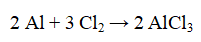
A) Al
B) Al and Cl2
C) Al and AlCl3
D) Cl2 and AlCl3
E) Cl2
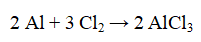
A) Al
B) Al and Cl2
C) Al and AlCl3
D) Cl2 and AlCl3
E) Cl2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
13
Which statement about strong acids is true?
A) Strong acids are weak electrolytes.
B) Strong acids are very concentrated.
C) Strong acids are mostly converted to ions when dissolved in water.
D) Citric acid is a strong acid.
E) Strong acids react only with strong bases.
A) Strong acids are weak electrolytes.
B) Strong acids are very concentrated.
C) Strong acids are mostly converted to ions when dissolved in water.
D) Citric acid is a strong acid.
E) Strong acids react only with strong bases.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
14
Which statement about neutralization reactions is true?
A) A weak acid cannot neutralize a strong base.
B) The net ionic equation for a neutralization reaction shows the formation of hydrogen gas
C) The net ionic equation for a neutralization reaction shows the formation of soluble salts.
D) The net ionic equation for a neutralization reaction shows the formation of water.
E) Organic acids neutralize bases by forming hydrogen gas.
A) A weak acid cannot neutralize a strong base.
B) The net ionic equation for a neutralization reaction shows the formation of hydrogen gas
C) The net ionic equation for a neutralization reaction shows the formation of soluble salts.
D) The net ionic equation for a neutralization reaction shows the formation of water.
E) Organic acids neutralize bases by forming hydrogen gas.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
15
Which statement about the reaction below is true, given large amounts of reactants? 
A) SrSO4 is soluble in water and will not precipitate.
B) NaCl is a spectator ion and will not precipitate.
C) SO42- is a spectator ion and will not precipitate.
D) Na+ is a spectator ion and will not precipitate.
E) All compounds in the reaction are soluble in water and no reaction occurs.

A) SrSO4 is soluble in water and will not precipitate.
B) NaCl is a spectator ion and will not precipitate.
C) SO42- is a spectator ion and will not precipitate.
D) Na+ is a spectator ion and will not precipitate.
E) All compounds in the reaction are soluble in water and no reaction occurs.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
16
What is the net ionic equation for the reaction of Ca(NO3)2 and Na2SO4?
A) 2Ca+ + SO42- Ca2SO4
B) Na+ + NO3- NaNO3
C) Ca2+ + SO42- CaSO4
D) Na2+ + 2NO3- Na(NO3)2
E) Ca+ + SO4- CaSO4
A) 2Ca+ + SO42- Ca2SO4
B) Na+ + NO3- NaNO3
C) Ca2+ + SO42- CaSO4
D) Na2+ + 2NO3- Na(NO3)2
E) Ca+ + SO4- CaSO4

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
17
What is the correct formula for the hydronium ion?
A) OH-
B) H3O+
C) H+
D) H-
E) NH4+
A) OH-
B) H3O+
C) H+
D) H-
E) NH4+

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
18
Which compounds will not dissolve in water in large amounts at 20 C? 
A) I & II
B) I & IV
C) II & III
D) III & IV

A) I & II
B) I & IV
C) II & III
D) III & IV

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following factors cause exchange reactions to occur?
A) formation of a gas, precipitate or water
B) formation of a gas or a precipitate
C) formation of a gas only
D) formation of a precipitate only
E) formation of water only
A) formation of a gas, precipitate or water
B) formation of a gas or a precipitate
C) formation of a gas only
D) formation of a precipitate only
E) formation of water only

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
20
Which compound will not dissolve in water in large amounts?
A) KNO3
B) NH4Cl
C) Ca(OH)2
D) AgCl
E) Ag2SO4
A) KNO3
B) NH4Cl
C) Ca(OH)2
D) AgCl
E) Ag2SO4

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
21
What is the oxidation number of O in H2O2?
A) -2
B) -1
C) 0
D) +1
E) +2
A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
22
What is reduced in the reaction below? 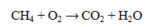
A) CH4
B) CO2
C) O2
D) H2O
E) O2 and CO2
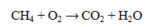
A) CH4
B) CO2
C) O2
D) H2O
E) O2 and CO2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
23
What is the oxidation number of O in Fe2O3?
A) +2
B) -2
C) -3
D) -5
E) +5
A) +2
B) -2
C) -3
D) -5
E) +5

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
24
Determine the volume of solution that contains 15.0 g of AgNO3 if the solution is 0.375 M.
A) 31.5 mL
B) 33.1 mL
C) 235 mL
D) 247 mL
E) 956 mL
A) 31.5 mL
B) 33.1 mL
C) 235 mL
D) 247 mL
E) 956 mL

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
25
How many moles of NaOH are present in 25.0 mL of a 0.1000 M NaOH solution?
A) 100 mol
B) 2.50 × 10-3 mol
C) 0.100 mol
D) 2.50 mol
E) 25.0 mol
A) 100 mol
B) 2.50 × 10-3 mol
C) 0.100 mol
D) 2.50 mol
E) 25.0 mol

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
26
A 50.0 mL sample of 0.108 M H2SO4 is diluted to 250.0 mL. What is its new molarity?
A) 0.0216 M
B) 0.108 M
C) 0.184 M
D) 0.461 M
E) 0.542 M
A) 0.0216 M
B) 0.108 M
C) 0.184 M
D) 0.461 M
E) 0.542 M

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
27
Which reaction will not occur?
A) Al(s) + SnCl2(aq) AlCl3(aq) + Sn(s)
B) H2(g) + AgNO3(aq) HNO3(aq) + Ag(s)
C) Mg(s) + CuSO4(aq) Cu(s) + MgSO4(aq)
D) Mg(s) + CaSO4(aq) MgSO4(aq) + Ca(s)
E) Ba(s) + HCl(aq) BaCl2(aq) + H2(g)
A) Al(s) + SnCl2(aq) AlCl3(aq) + Sn(s)
B) H2(g) + AgNO3(aq) HNO3(aq) + Ag(s)
C) Mg(s) + CuSO4(aq) Cu(s) + MgSO4(aq)
D) Mg(s) + CaSO4(aq) MgSO4(aq) + Ca(s)
E) Ba(s) + HCl(aq) BaCl2(aq) + H2(g)

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
28
Which element is most reactive?
A) Cu
B) Fe
C) K
D) Mg
E) Pb
A) Cu
B) Fe
C) K
D) Mg
E) Pb

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following represents oxidation?
A) 2 H+ + 2 e- H2
B) F2 + 2 e- 2 F-
C) Mg2+ + 2 e- Mg
D) Na Na+ + e-
E) Ni2+ + 2 e- Ni
A) 2 H+ + 2 e- H2
B) F2 + 2 e- 2 F-
C) Mg2+ + 2 e- Mg
D) Na Na+ + e-
E) Ni2+ + 2 e- Ni

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
30
What is the oxidation number of N in NO?
A) -2
B) -1
C) 0
D) +1
E) +2
A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
31
Which substance is reduced in the reaction below? 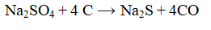
A) CO
B) S
C) Na2S
D) Na2SO4
E) none of these
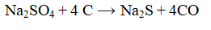
A) CO
B) S
C) Na2S
D) Na2SO4
E) none of these

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following represents reduction?
A) Al Al3+ + 3 e-
B) O2 + 4 e- 2 O2-
C) Fe Fe2+ + 2 e-
D) Na Na+ + e-
E) H2 2 H+ + 2 e-
A) Al Al3+ + 3 e-
B) O2 + 4 e- 2 O2-
C) Fe Fe2+ + 2 e-
D) Na Na+ + e-
E) H2 2 H+ + 2 e-

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
33
What is the molarity of a solution that results when 21.3 g of (NH4)3PO4 is dissolved in water and diluted to exactly 250.0 mL?
A) 1.61 × 10-4 M
B) 0.357 M
C) 0.572 M
D) 2.28 M
E) 85.2 M
A) 1.61 × 10-4 M
B) 0.357 M
C) 0.572 M
D) 2.28 M
E) 85.2 M

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
34
What is oxidized in the reaction below? 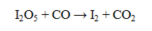
A) CO
B) CO2
C) I2
D) I2O5
E) CO and CO2
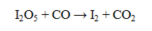
A) CO
B) CO2
C) I2
D) I2O5
E) CO and CO2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
35
What is the oxidation number of P in PO43-?
A) +5
B) +3
C) -2
D) -3
E) -5
A) +5
B) +3
C) -2
D) -3
E) -5

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
36
If a 45.0 mL sample of 2.20 M Na2SO4 is diluted to yield a final solution that is 0.110 M in sodium ions, what is the volume of the final solution?
A) 110 mL
B) 450 mL
C) 900 mL
D) 1800 mL
E) 4500 mL
A) 110 mL
B) 450 mL
C) 900 mL
D) 1800 mL
E) 4500 mL

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
37
What is the oxidation number of N in NH4+?
A) -3
B) -1
C) 0
D) +1
E) +3
A) -3
B) -1
C) 0
D) +1
E) +3

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
38
Which substance is oxidized in the reaction below? 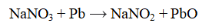
A) Na+
B) PbO
C) NaNO3
D) NaNO2
E) none of these
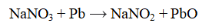
A) Na+
B) PbO
C) NaNO3
D) NaNO2
E) none of these

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
39
Determine the ammonium ion concentration of a solution that results when 4.53 g of (NH4)2SO4 is dissolved in water and diluted to exactly 100.0 mL.
A) 0.343 M
B) 0.686 M
C) 1.03 M
D) 1.37 M
E) 2.51 M
A) 0.343 M
B) 0.686 M
C) 1.03 M
D) 1.37 M
E) 2.51 M

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
40
Which element is least reactive?
A) Al
B) Cu
C) Zn
D) Pb
E) Fe
A) Al
B) Cu
C) Zn
D) Pb
E) Fe

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
41
Oxalic acid, H2C2O4, reacts with Y(NO3)3 as shown by the equation below. What weight of yttrium oxalate is produced from 50.0 mL of 0.265 M Y(NO3)3 and excess oxalic acid? Assume that all of the yttrium oxalate is insoluble. 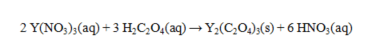
A) 1.46 g
B) 1.82 g
C) 2.93 g
D) 3.64 g
E) 5.85 g
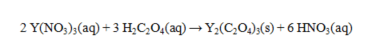
A) 1.46 g
B) 1.82 g
C) 2.93 g
D) 3.64 g
E) 5.85 g

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
42
Give an example of a strong base.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
43
When an element is _____________, it loses electrons.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
44
How many grams of PbI2 will precipitate from the reaction of 14.0 mL of 0.190 M Pb(NO3)2 with excess KI solution? Assume that all of the PbI2 is insoluble.
A) 1.23 g
B) 1.33 g
C) 2.66 g
D) 3.69 g
E) 6260 g
A) 1.23 g
B) 1.33 g
C) 2.66 g
D) 3.69 g
E) 6260 g

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the methods described below will yield 500 mL of a 0.100 M KMnO4 solution?
A) Add exactly 500 mL of water to 7.90 g of KMnO4.
B) Add exactly 500 mL of water to KMnO4.
C) Dissolve 7.90 g of KMnO4 in water and dilute to exactly 500 mL.
D) Dissolve 15.8 g KMnO4 in water and dilute to exactly 500 mL.
E) Dilute 220 mL of 1.00 M KMnO4 to exactly 500 mL.
A) Add exactly 500 mL of water to 7.90 g of KMnO4.
B) Add exactly 500 mL of water to KMnO4.
C) Dissolve 7.90 g of KMnO4 in water and dilute to exactly 500 mL.
D) Dissolve 15.8 g KMnO4 in water and dilute to exactly 500 mL.
E) Dilute 220 mL of 1.00 M KMnO4 to exactly 500 mL.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
46
Determine the mass of BaSO4 that is produced by the reaction of 45.0 mL of 0.155 M H2SO4 and 60.0 mL of 0.125 M BaCl2. Assume that BaSO4 is totally insoluble. 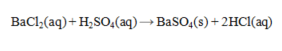
A) 1.45 g
B) 1.62 g
C) 1.79 g
D) 3.24 g
E) 0.775 g
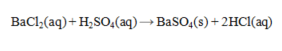
A) 1.45 g
B) 1.62 g
C) 1.79 g
D) 3.24 g
E) 0.775 g

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
47
A 25.00 mL sample of HCl solution is neutralized by exactly 41.63 mL of 0.1363 M NaOH. What is the molarity of the HCl solution?
A) 0.2270 M
B) 0.2726 M
C) 0.06815 M
D) 0.1135 M
E) 0.05675 M
A) 0.2270 M
B) 0.2726 M
C) 0.06815 M
D) 0.1135 M
E) 0.05675 M

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
48
A 64.05 mL sample of 0.250 M CH3COOH is to be exactly neutralized with 0.500 M KOH. How many milliliters of KOH will be required?
A) 64.1 mL
B) 32.0 mL
C) 128 mL
D) 16.0 mL
E) 8.01 mL
A) 64.1 mL
B) 32.0 mL
C) 128 mL
D) 16.0 mL
E) 8.01 mL

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
49
Molarity is a unit of solution concentration expressed in moles per _____________.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
50
A 25.00 mL sample of H3PO4 solution is neutralized by exactly 54.93 mL of 0.04345 M Ca(OH)2. What is the molarity of the H3PO4 solution?
A) 0.0636 M
B) 0.09546 M
C) 0.1432 M
D) 0.2148 M
E) 0.2897 M
A) 0.0636 M
B) 0.09546 M
C) 0.1432 M
D) 0.2148 M
E) 0.2897 M

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
51
A 65.00 mL sample of HNO3 solution is neutralized by exactly 34.16 mL of 0.5841 M Ba(OH)2. What is the molarity of the HNO3 solution?
A) 0.1535 M
B) 0.2880 M
C) 0.3110 M
D) 0.6139 M
E) 2.594 M
A) 0.1535 M
B) 0.2880 M
C) 0.3110 M
D) 0.6139 M
E) 2.594 M

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
52
How many moles of ions are in 285 mL of 0.0150 M MgCl2?
A) 4.128 × 10-3 mol
B) 5.26 × 10-2 mol
C) 1.28 × 10-2 mol
D) 1.05 × 10-1 mol
E) 5.26 × 10-1 mol
A) 4.128 × 10-3 mol
B) 5.26 × 10-2 mol
C) 1.28 × 10-2 mol
D) 1.05 × 10-1 mol
E) 5.26 × 10-1 mol

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
53
Ammonia and sulfuric acid react according to the equation given below. How many milliliters of 0.110 M sulfuric acid are required to exactly neutralize 25.0 mL of 0.0840 M NH3 solution?
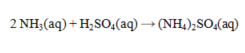
A) 1.46 mL
B) 1.82 mL
C) 3.64 mL
D) 5.85 mL
E) 9.55 mL
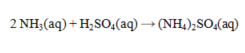
A) 1.46 mL
B) 1.82 mL
C) 3.64 mL
D) 5.85 mL
E) 9.55 mL

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
54
Hydrochloric acid solutions are often standardized by the reaction below. How many grams of CaCO3 are required to exactly react with 50.0 mL of 0.155 M HCl? 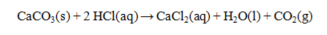
A) 0.194 g
B) 0.283 g
C) 0.341 g
D) 0.387 g
E) 0.566 g
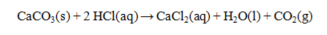
A) 0.194 g
B) 0.283 g
C) 0.341 g
D) 0.387 g
E) 0.566 g

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
55
A solution is prepared by dissolving 20.0 g of NaI in enough water to make 300.0 mL of solution. How many moles of ions are in 25.0 mL of this solution?
A) 0.0111 mol
B) 0.0222 mol
C) 0.0445 mol
D) 0.111 mol
E) 0.445 mol
A) 0.0111 mol
B) 0.0222 mol
C) 0.0445 mol
D) 0.111 mol
E) 0.445 mol

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
56
How many grams of KCl are in 125.0 mL of 0.375 M KCl?
A) 3.49 g
B) 0.0469 g
C) 3.49 × 10-3 g
D) 46.9 g
E) 0.938 g
A) 3.49 g
B) 0.0469 g
C) 3.49 × 10-3 g
D) 46.9 g
E) 0.938 g

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
57
A 25.00 mL sample of HCl solution is neutralized by exactly 31.22 mL of 0.08152 M Ca(OH)2. What is the molarity of the HCl solution?
A) 0.08152 M
B) 0.1018 M
C) 0.2036 M
D) 0.1021 M
E) 0.09453 M
A) 0.08152 M
B) 0.1018 M
C) 0.2036 M
D) 0.1021 M
E) 0.09453 M

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
58
A solution is made by dissolving 60.0 g of AlCl3 in enough water to make 250.0 mL of solution. How many moles of ions are in 5.00 mL of solution?
A) 3.60 × 10-2 mol
B) 1.01 × 10-3 mol
C) 1.25 × 10-3 mol
D) 5.00 × 10-3 mol
E) 9.00 × 10-3 mol
A) 3.60 × 10-2 mol
B) 1.01 × 10-3 mol
C) 1.25 × 10-3 mol
D) 5.00 × 10-3 mol
E) 9.00 × 10-3 mol

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
59
A neutralization reaction involves the reaction of a(n) _____________ with a(n) _____________.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
60
How many moles of ions are in a 250.0 mL sample of 0.150 M NaCl?

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
61
Write the net ionic equation for the reaction given.
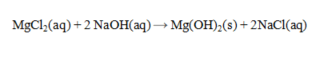
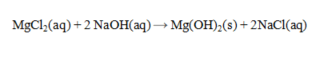

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
62
Match between columns

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
63
A 25.0 mL solution of HNO3 is reacted with 0.1125 M NaOH. The endpoint is reached when 57.8 mL of NaOH has been added. What is the molarity of HNO3?

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
64
A solution is made by dissolving 15.0 g of CaCl2 in enough water to make exactly 500.0 mL of solution. What is the molarity of the solution?

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
65
A chemical reaction for which spectator ions are deleted is called a(n) _____________ ionic equation.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
66
Match the following:
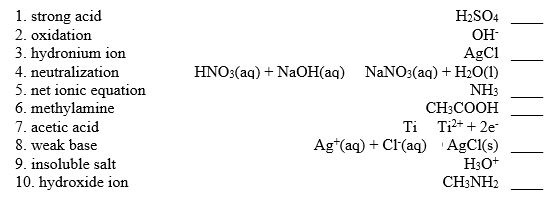
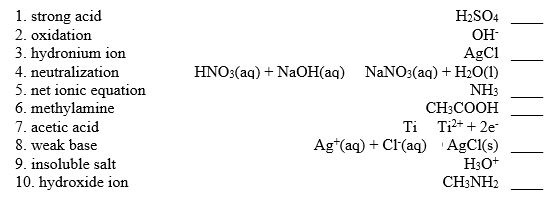

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
66
Which substance is an oxidizing agent in the reaction below?
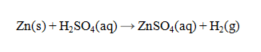
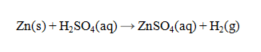

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck


