Exam 5: Chemical Reactions
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
A 50.0 mL sample of 0.108 M H2SO4 is diluted to 250.0 mL. What is its new molarity?
Free
(Multiple Choice)
4.7/5  (27)
(27)
Correct Answer:
A
Which of the following is a reducing agent?
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
B
Determine the mass of BaSO4 that is produced by the reaction of 45.0 mL of 0.155 M H2SO4 and 60.0 mL of 0.125 M BaCl2. Assume that BaSO4 is totally insoluble. 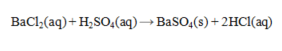
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
B
Which statement about the reaction below is true, given large amounts of reactants? 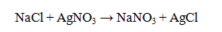
(Multiple Choice)
5.0/5  (36)
(36)
A 65.00 mL sample of HNO3 solution is neutralized by exactly 34.16 mL of 0.5841 M Ba(OH)2. What is the molarity of the HNO3 solution?
(Multiple Choice)
4.9/5  (36)
(36)
A 64.05 mL sample of 0.250 M CH3COOH is to be exactly neutralized with 0.500 M KOH. How many milliliters of KOH will be required?
(Multiple Choice)
4.9/5  (32)
(32)
Determine the ammonium ion concentration of a solution that results when 4.53 g of (NH4)2SO4 is dissolved in water and diluted to exactly 100.0 mL.
(Multiple Choice)
4.9/5  (33)
(33)
Oxalic acid, H2C2O4, reacts with Y(NO3)3 as shown by the equation below. What weight of yttrium oxalate is produced from 50.0 mL of 0.265 M Y(NO3)3 and excess oxalic acid? Assume that all of the yttrium oxalate is insoluble. 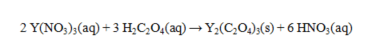
(Multiple Choice)
4.8/5  (25)
(25)
Hydrochloric acid solutions are often standardized by the reaction below. How many grams of CaCO3 are required to exactly react with 50.0 mL of 0.155 M HCl? 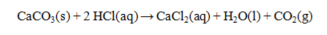
(Multiple Choice)
4.8/5  (35)
(35)
Which of the methods described below will yield 500 mL of a 0.100 M KMnO4 solution?
(Multiple Choice)
4.8/5  (36)
(36)
Which one or two substances are reduced in the reaction below? 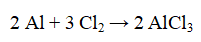
(Multiple Choice)
4.8/5  (36)
(36)
How many moles of ions are in a 250.0 mL sample of 0.150 M NaCl?
(Short Answer)
4.8/5  (39)
(39)
Showing 1 - 20 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)