Deck 19: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/53
Play
Full screen (f)
Deck 19: Nuclear Chemistry
1
In the reaction shown, the radiation produced is a(n) 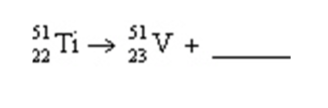
A) alpha particle.
B) positron.
C) beta particle.
D) neutron.
E) gamma ray.
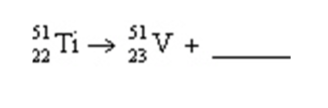
A) alpha particle.
B) positron.
C) beta particle.
D) neutron.
E) gamma ray.
beta particle.
2
The phenomenon of radioactivity was first observed by
A) Becquerel.
B) the Curies.
C) Marconi.
D) Roentgen.
E) Rutherford.
A) Becquerel.
B) the Curies.
C) Marconi.
D) Roentgen.
E) Rutherford.
Becquerel.
3
Gamma radiation is best described as
A) neutral particles that weigh approximately one atomic mass unit.
B) positive particles that are identical to the nucleus of an atom of 4He.
C) electrons ejected at high speeds from a radioactive nucleus.
D) a form of electromagnetic radiation.
E) high-speed particles similar in size to an electron, but oppositely charged.
A) neutral particles that weigh approximately one atomic mass unit.
B) positive particles that are identical to the nucleus of an atom of 4He.
C) electrons ejected at high speeds from a radioactive nucleus.
D) a form of electromagnetic radiation.
E) high-speed particles similar in size to an electron, but oppositely charged.
a form of electromagnetic radiation.
4
Radioactivity is emission of radiation which
A) is always accompanied by a chemical process.
B) is always preceded by a chemical process.
C) is initiated by absorption of radiation.
D) is initiated by absorption of heat.
E) is spontaneous.
A) is always accompanied by a chemical process.
B) is always preceded by a chemical process.
C) is initiated by absorption of radiation.
D) is initiated by absorption of heat.
E) is spontaneous.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
5
The reaction represented by the description "An atom of titanium-45 decays by beta emission" is
A)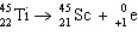
B)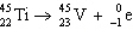
C)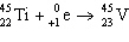
D)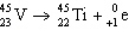
E)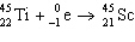
A)
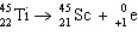
B)
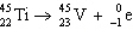
C)
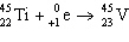
D)
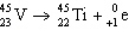
E)
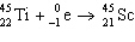

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
6
The most penetrating type of radioactivity is ____, and the least penetrating is ____.
A) alpha particles; gamma rays
B) beta particles; alpha particles
C) beta particles; gamma rays
D) gamma rays; alpha particles
E) gamma rays; beta particles
A) alpha particles; gamma rays
B) beta particles; alpha particles
C) beta particles; gamma rays
D) gamma rays; alpha particles
E) gamma rays; beta particles

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
7
Alpha radiation consists of
A) electromagnetic radiation
B) electrons ejected from inner orbitals
C) electrons ejected from nuclei
D) hydrogen nuclei ejected from nuclei
E) helium nuclei ejected from nuclei
A) electromagnetic radiation
B) electrons ejected from inner orbitals
C) electrons ejected from nuclei
D) hydrogen nuclei ejected from nuclei
E) helium nuclei ejected from nuclei

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
8
In the reaction shown, Cm is known as the ____ nucleus, and Pu as the ____ nucleus. 
A) target; projectile
B) projectile; target
C) alpha; beta
D) parent; daughter
E) daughter; parent

A) target; projectile
B) projectile; target
C) alpha; beta
D) parent; daughter
E) daughter; parent

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
9
The reaction represented by the description "An atom of lead-210 decays by emission of an alpha particle" is
A)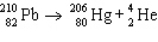
B)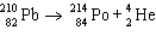
C)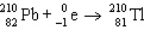
D)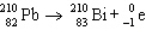
E)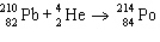
A)
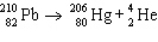
B)
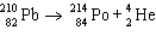
C)
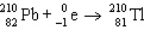
D)
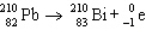
E)
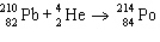

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
10
Alpha particles are best described as
A) neutral particles that weigh approximately one atomic mass unit.
B) positive particles that are identical to the nucleus of an atom of 4He.
C) electrons ejected at high speeds from a radioactive nucleus.
D) high-speed particles similar in size to an electron, but oppositely charged.
E) a form of electromagnetic radiation.
A) neutral particles that weigh approximately one atomic mass unit.
B) positive particles that are identical to the nucleus of an atom of 4He.
C) electrons ejected at high speeds from a radioactive nucleus.
D) high-speed particles similar in size to an electron, but oppositely charged.
E) a form of electromagnetic radiation.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
11
Beta particles are best described as ____, ejected at high speeds from a radioactive nucleus.
A) protons
B) particles similar in size to an electron, but oppositely charged
C) electrons
D) neutral particles weighing approximately one atomic mass unit
E) positive particles identical to the nucleus of an atom of 4He
A) protons
B) particles similar in size to an electron, but oppositely charged
C) electrons
D) neutral particles weighing approximately one atomic mass unit
E) positive particles identical to the nucleus of an atom of 4He

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
12
A radioactive isotope that decomposes by alpha particle emission loses
A) a proton
B) a neutron
C) an electron
D) a positron
E) a helium nucleus
A) a proton
B) a neutron
C) an electron
D) a positron
E) a helium nucleus

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
13
Which of these does not correspond to a real radioactive decay process?
A)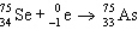
B)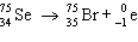
C)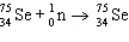
D) neither a nor b
E) neither a nor c
A)
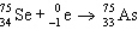
B)
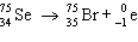
C)
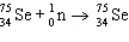
D) neither a nor b
E) neither a nor c

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
14
A radioactive isotope that decomposes by positron emission loses
A) an electron
B) a proton
C) a helium nucleus
D) a particle similar to an electron, but positively charged
E) a positively-charged neutron
A) an electron
B) a proton
C) a helium nucleus
D) a particle similar to an electron, but positively charged
E) a positively-charged neutron

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
15
A radioactive isotope that decomposes by beta particle emission loses
A) a positron
B) an electron
C) a neutron
D) a proton
E) a helium nucleus
A) a positron
B) an electron
C) a neutron
D) a proton
E) a helium nucleus

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
16
A radioactive isotope that decomposes by K-electron capture emission
A) combines one of its electrons with one of its protons creating an additional neutron
B) gains an electron from its environment
C) gains a helium nucleus
D) gains a particle similar to an electron, but positively charged
E) gains a negatively-charged neutron
A) combines one of its electrons with one of its protons creating an additional neutron
B) gains an electron from its environment
C) gains a helium nucleus
D) gains a particle similar to an electron, but positively charged
E) gains a negatively-charged neutron

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
17
If polonium-210 emits an alpha particle, the other product will be
A) lead-206.
B) mercury-204.
C) mercury-206.
D) polonium-208.
E) radon-206.
A) lead-206.
B) mercury-204.
C) mercury-206.
D) polonium-208.
E) radon-206.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
18
Beta emission can best be described as involving
A) emission from an unstable nucleus of a fragment containing two protons and two neutrons
B) annihilation of a beta particle by a positron
C) capture of an electron by the nucleus, and transformation of a proton into a neutron
D) transformation of a neutron into a electron, and a proton that is ejected from the nucleus
E) transformation of a neutron into a proton, and an electron that is ejected from the nucleus
A) emission from an unstable nucleus of a fragment containing two protons and two neutrons
B) annihilation of a beta particle by a positron
C) capture of an electron by the nucleus, and transformation of a proton into a neutron
D) transformation of a neutron into a electron, and a proton that is ejected from the nucleus
E) transformation of a neutron into a proton, and an electron that is ejected from the nucleus

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
19
The reaction represented by the description "An atom of metastable strontium-87 undergoes gamma decay" is
A)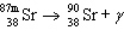
B)
C)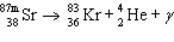
D)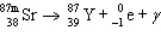
E)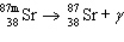
A)
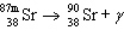
B)

C)
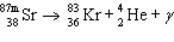
D)
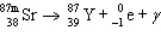
E)
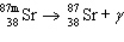

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
20
In the reaction shown, the radiation produced is a(n) 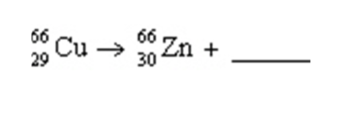
A) alpha particle
B) beta particle
C) gamma ray
D) neutron
E) positron
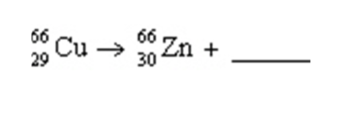
A) alpha particle
B) beta particle
C) gamma ray
D) neutron
E) positron

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
21
The fission reaction exploited in nuclear power stations involves
A) neutron bombardment of uranium-235.
B) neutron bombardment of uranium-238.
C) neutron bombardment of plutonium-239.
D) x-ray irradiation of plutonium-241.
E) x-ray irradiation of uranium-235.
A) neutron bombardment of uranium-235.
B) neutron bombardment of uranium-238.
C) neutron bombardment of plutonium-239.
D) x-ray irradiation of plutonium-241.
E) x-ray irradiation of uranium-235.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
22
90Sr is an isotope produced from atmospheric testing of nuclear bombs. If nuclear testing was stopped in 1960, what percentage of radioactivity due to 90Sr remained in 2000? The half-life of 90Sr is 28.5 years.
A) 62.2 %
B) 37.8 %
C) 12.3 %
D) 0.85 %
E) virtually 0 %
A) 62.2 %
B) 37.8 %
C) 12.3 %
D) 0.85 %
E) virtually 0 %

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
23
If pure polonium-210 (k = 5.02 × 10-3 day-1) were used in a crime, based on radioactive disintegration alone, how long would it take for half the original isotope to disappear?
A) 1.44 × 103 minutes
B) 69.3 hours
C) 138 days
D) 2.9 years
E) 210 years
A) 1.44 × 103 minutes
B) 69.3 hours
C) 138 days
D) 2.9 years
E) 210 years

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
24
Beyond which element do all other elements have nuclei that are unstable and radioactive?
A) Nb-93
B) In-115
C) La-139
D) W-184
E) Bi-209
A) Nb-93
B) In-115
C) La-139
D) W-184
E) Bi-209

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
25
Radioactive decay exhibits
A) zeroth-order kinetics
B)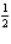 -th order kinetics
-th order kinetics
C) first-order kinetics
D) second-order kinetics
E) third-order kinetics
A) zeroth-order kinetics
B)
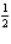 -th order kinetics
-th order kineticsC) first-order kinetics
D) second-order kinetics
E) third-order kinetics

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
26
Assuming two samples of radioisotopes both contain the same number of atoms, the activity of the shorter-half-life isotope would be initially ____ and decay ____.
A) lower; more slowly
B) the same; more slowly
C) higher; more slowly
D) higher; more quickly
E) lower; more quickly
A) lower; more slowly
B) the same; more slowly
C) higher; more slowly
D) higher; more quickly
E) lower; more quickly

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
27
Samples of the following isotopes in pure form, each containing the same number of atoms, are taken. After one year, which sample would still contain the most atoms of the original substance?
A) sodium-24 (half-life 15 hours)
B) strontium-90 (half-life 29 years)
C) iodine-131 (half-life 8 days)
D) lead-210 (half-life 22 years)
E) thallium-206 (half-life 4 min)
A) sodium-24 (half-life 15 hours)
B) strontium-90 (half-life 29 years)
C) iodine-131 (half-life 8 days)
D) lead-210 (half-life 22 years)
E) thallium-206 (half-life 4 min)

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
28
Which statement concerning fusion is correct?
A) Extremely high temperatures are not required.
B) A plasma of unbound nuclei and electrons must be formed.
C) Large nuclei are needed as reactants for fusion reactions.
D) Fusion reactions require large amounts of energy to sustain them.
E) Products of fusion reactions are always radioactive.
A) Extremely high temperatures are not required.
B) A plasma of unbound nuclei and electrons must be formed.
C) Large nuclei are needed as reactants for fusion reactions.
D) Fusion reactions require large amounts of energy to sustain them.
E) Products of fusion reactions are always radioactive.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
29
All elements in the Periodic Table beyond ____ have only radioactive isotopes; these mostly decay by ____ emission.
A) Bi; alpha
B) Po; beta
C) Ra; alpha
D) U; beta
E) Pu; gamma
A) Bi; alpha
B) Po; beta
C) Ra; alpha
D) U; beta
E) Pu; gamma

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
30
The half-life of radon-222 is 2.8 days. How many days would it take for the activity due to radon in a tightly closed building to decrease to 2.8 % of its original value?
A) 1.0
B) 8.9
C) 14
D) 17.8
E) 100
A) 1.0
B) 8.9
C) 14
D) 17.8
E) 100

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
31
A radioactive material has a half-life of 2.5 hours. The activity of a sample was measured to be 375 cps. What was the activity of the sample 6 hr, 15 min earlier?
A) 60 cps
B) 66 cps
C) 8.6 × 102 cps
D) 2.1 × 103 cps
E) 4.9 × 103 cps
A) 60 cps
B) 66 cps
C) 8.6 × 102 cps
D) 2.1 × 103 cps
E) 4.9 × 103 cps

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
32
Which pair of elements could not be produced from neutron bombardment of uranium-235 if 2 additional neutrons are also produced?
A) cesium-143 and rubidium-91
B) bromine-87 and lanthanum-147
C) zirconium-97 and barium-139
D) tellurium-137 and zirconium-97
E) iodine-141 and yttrium-93
A) cesium-143 and rubidium-91
B) bromine-87 and lanthanum-147
C) zirconium-97 and barium-139
D) tellurium-137 and zirconium-97
E) iodine-141 and yttrium-93

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
33
The half-life of radon-222 is 2.8 days. A homeowner used a radon test-kit to sample the air in his home, but forgot to send it for processing for 30 days. If the level of radon was actually 100 counts, what value would be reported by the test lab?
A) 94 counts
B) 17 counts
C) 9.3 counts
D) 1.5 counts
E) 0.060 counts
A) 94 counts
B) 17 counts
C) 9.3 counts
D) 1.5 counts
E) 0.060 counts

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
34
How many Bq correspond to an activity of 7.4 mCi? (1 mCi = 3.7 × 107 dps and 1 Bq = 1 dps.)
A) 5.0 × 10-6
B) 2.0 × 10-5
C) 2.0 × 105
D) 2.7 × 105
E) 5.0 × 106
A) 5.0 × 10-6
B) 2.0 × 10-5
C) 2.0 × 105
D) 2.7 × 105
E) 5.0 × 106

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
35
Which element has only radioactive isotopes?
A) Al
B) La
C) Li
D) Lr
E) Ir
A) Al
B) La
C) Li
D) Lr
E) Ir

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
36
All of these are common modes of radioactive decay except
A) positron capture
B) positron emission
C) electron capture
D) beta emission
E) alpha emission
A) positron capture
B) positron emission
C) electron capture
D) beta emission
E) alpha emission

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
37
The force holding the protons and neutrons together in a nucleus is known as
A) binding energy
B) gamma radiation
C) electrostatic attraction
D) gravity
E) mass defect
A) binding energy
B) gamma radiation
C) electrostatic attraction
D) gravity
E) mass defect

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
38
Based solely on the fact that, for elements beyond Bi-209 all nuclei are unstable and radioactive, we can conclusively determine that
A) in order to be radioactive, an element must have a mass of at least 210
B) polonium-210 is radioactive, but polonium-208 and polonium-209 are not radioactive
C) both uranium-235 and uranium-238 are radioactive
D) phosporus-32 and sulfur-35 are not radioactive
E) lead does not have radioactive isotopes
A) in order to be radioactive, an element must have a mass of at least 210
B) polonium-210 is radioactive, but polonium-208 and polonium-209 are not radioactive
C) both uranium-235 and uranium-238 are radioactive
D) phosporus-32 and sulfur-35 are not radioactive
E) lead does not have radioactive isotopes

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
39
The rule that the mass number of stable isotopes is always at least twice the atomic number is true for all nuclides except hydrogen-1 and
A) beryllium-10
B) helium-3
C) magnesium-28
D) strontium-90
E) vanadium-52
A) beryllium-10
B) helium-3
C) magnesium-28
D) strontium-90
E) vanadium-52

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
40
A process for disposing of nuclear waste by converting it to an inert glass-like substance is called
A) extraction.
B) incineration.
C) regeneration.
D) reprocessing.
E) vitrification.
A) extraction.
B) incineration.
C) regeneration.
D) reprocessing.
E) vitrification.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
41
How many of these do not involve nuclear fusion: a nuclear power station, the sun, Positron Emission Tomography, a hydrogen bomb?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
42
The radiation unit equal to 1 J of energy deposited per kg of tissue is the
A) becquerel.
B) curie.
C) gray.
D) roentgen.
E) rem.
A) becquerel.
B) curie.
C) gray.
D) roentgen.
E) rem.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
43
Which unit allows for the varying effects of different types of radiation on human tissue?
A) curie
B) gray
C) rad
D) rem
E) roentgen
A) curie
B) gray
C) rad
D) rem
E) roentgen

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
44
The SI unit of effective radiation dose is the
A) gray
B) rad
C) rem
D) roentgen
E) sievert
A) gray
B) rad
C) rem
D) roentgen
E) sievert

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
45
One of the routes for radioactive decay of U-238 to Pb-206 involves the successive emission of alpha, two beta, four alpha, beta, alpha, beta, alpha, beta, alpha, and beta particles.
a. By consideration of the atomic and mass numbers of all species in this reaction, show that the statement above is consistent.
b. Identify the first five isotopes encountered during this process.
a. By consideration of the atomic and mass numbers of all species in this reaction, show that the statement above is consistent.
b. Identify the first five isotopes encountered during this process.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
46
Explain the meaning of the term critical mass in relation to the use of a chain reaction for the production of nuclear power.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following processes is used in nuclear power plants for the production of electricity?
A) alpha particle capture
B) K-electron capture
C) nuclear fission
D) nuclear fusion
E) electron emission
A) alpha particle capture
B) K-electron capture
C) nuclear fission
D) nuclear fusion
E) electron emission

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
48
Which of these is the smallest contributor to background radiation exposure in the U.S.?
A) cosmic radiation
B) radon
C) x-rays
D) uranium
E) nuclear wastes
A) cosmic radiation
B) radon
C) x-rays
D) uranium
E) nuclear wastes

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
49
Calculate the binding energy per nucleon for bromine-81, with a mass of 80.9163 amu. The mass of a proton is 1.007825 amu and the mass of a neutron is 1.008665 amu.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
50
Major technical obstacle(s) to the achievement of a controlled fusion reaction is (are)
I. attaining a temperature of at least 1.0 ´ 108 K
II. containment of the plasma long enough to generate a net output of energy
III. recovering the energy in some useable form
A) I only
B) II only
C) III only
D) I, II, and III
E) I and II only
I. attaining a temperature of at least 1.0 ´ 108 K
II. containment of the plasma long enough to generate a net output of energy
III. recovering the energy in some useable form
A) I only
B) II only
C) III only
D) I, II, and III
E) I and II only

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
51
On a plot of number of neutrons as a function of number of protons, the stable (non-radioactive) isotopes form a "peninsula of stability". Isotopes with a greater or lesser number of neutrons than this, and all isotopes beyond a certain number of protons, are radioactive. Briefly describe the most common type of radioactive decay exhibited by each of these groups.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
52
What is the largest single contributor to background radiation in the United States?
A) cosmic
B) medical X-rays
C) consumer products
D) nuclear power plants
E) radon
A) cosmic
B) medical X-rays
C) consumer products
D) nuclear power plants
E) radon

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
53
The rad is a radiation unit that describes the
A) multiples of the base unit of radiation
B) amount of energy absorbed per kilogram of tissue
C) amount of energy deposited per gram of tissue
D) energy equivalent in mammals of energy absorbed per gram of tissue
E) number of radioactive decays in one second
A) multiples of the base unit of radiation
B) amount of energy absorbed per kilogram of tissue
C) amount of energy deposited per gram of tissue
D) energy equivalent in mammals of energy absorbed per gram of tissue
E) number of radioactive decays in one second

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck


