Deck 18: Electrochemistry and Its Applications
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/54
Play
Full screen (f)
Deck 18: Electrochemistry and Its Applications
1
The unit used to measure electrical current is the
A) ampere
B) coulomb
C) faraday
D) joule
E) volt
A) ampere
B) coulomb
C) faraday
D) joule
E) volt
ampere
2
The unit used to measure electromotive force (emf) is the
A) horsepower
B) electromagnetic Faraday
C) volt
D) coulomb
E) ampere
A) horsepower
B) electromagnetic Faraday
C) volt
D) coulomb
E) ampere
horsepower
3
Consider an electrochemical cell as shown, with Zn in ZnCl2(aq) and Cu in Cu(NO3)2(aq), and a salt bridge containing KNO3(aq). The overall chemical reaction is 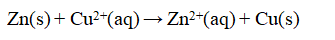
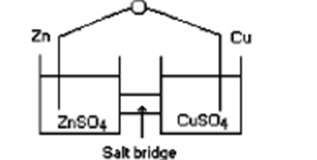
Which statement is correct?
A) one mole of electrons is transferred in this reaction
B) copper is oxidized at the anode
C) electrons travel from the Zn electrode to the Cu electrode
D) this is an example of a concentration cell
E) zinc is reduced at the cathode
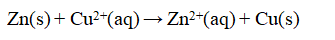

Which statement is correct?
A) one mole of electrons is transferred in this reaction
B) copper is oxidized at the anode
C) electrons travel from the Zn electrode to the Cu electrode
D) this is an example of a concentration cell
E) zinc is reduced at the cathode
electrons travel from the Zn electrode to the Cu electrode
4
Which cell notation represents a battery constructed using zinc and iron electrodes with electrons flowing from zinc to iron?
A) Fe3+(aq) | Fe2+(aq) || Zn(s) | Zn2+(aq)
B) Fe3+(aq) | Fe(s) || Zn(s) | Zn2+(aq)
C) Zn(s) | Zn2+(aq) || Fe3+(aq) | Fe2+(aq)
D) Zn(s) | Zn2+(aq) || Fe3+(aq) | Fe(s)
E) Zn(s) | Zn2+(aq) || Fe(s) | Fe3+(aq)
A) Fe3+(aq) | Fe2+(aq) || Zn(s) | Zn2+(aq)
B) Fe3+(aq) | Fe(s) || Zn(s) | Zn2+(aq)
C) Zn(s) | Zn2+(aq) || Fe3+(aq) | Fe2+(aq)
D) Zn(s) | Zn2+(aq) || Fe3+(aq) | Fe(s)
E) Zn(s) | Zn2+(aq) || Fe(s) | Fe3+(aq)

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
5
In the salt bridge in an electrochemical cell,
A) negatively charged ions migrate towards the anode compartment
B) positively charged ions migrate towards the anode compartment
C) negatively charged ions migrate towards the cathode compartment
D) both b and c
E) neither a nor c
A) negatively charged ions migrate towards the anode compartment
B) positively charged ions migrate towards the anode compartment
C) negatively charged ions migrate towards the cathode compartment
D) both b and c
E) neither a nor c

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
6
In the anode compartment of a simple electrochemical cell the electrode is being ____, and ____ are flowing in from the salt bridge.
A) oxidized; anions
B) oxidized; cations
C) oxidized; electrons
D) reduced; cations
E) reduced; anions
A) oxidized; anions
B) oxidized; cations
C) oxidized; electrons
D) reduced; cations
E) reduced; anions

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
7
Which statement is not correct?
A) an electrochemical cell can extract electrical energy from a reactant-favored chemical reaction
B) all electrochemical cells have at least two electrodes
C) a voltaic cell is a type of electrochemical cell
D) reduction occurs at the cathode in an electrochemical cell
E) a battery is an example of an electrochemical cell
A) an electrochemical cell can extract electrical energy from a reactant-favored chemical reaction
B) all electrochemical cells have at least two electrodes
C) a voltaic cell is a type of electrochemical cell
D) reduction occurs at the cathode in an electrochemical cell
E) a battery is an example of an electrochemical cell

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
8
Select the material containing the element with the highest oxidation number and determine how many electrons are required to reduce that element to an oxidation number of zero. 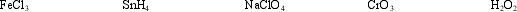
A) 8
B) 7
C) 6
D) 4
E) 3
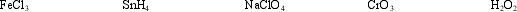
A) 8
B) 7
C) 6
D) 4
E) 3

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
9
Which change does not indicate a reduction?
A) decrease in oxidation number
B) gain of electrons
C) electrons as reactants
D) reactant acting as a reducing agent
E) pure oxygen becoming oxide ion
A) decrease in oxidation number
B) gain of electrons
C) electrons as reactants
D) reactant acting as a reducing agent
E) pure oxygen becoming oxide ion

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
10
If cadmium metal and the Fe(III) ion are mixed in aqueous solution, a solution containing Cd(II) and Fe(II) results. The balanced equation for this process is
A) Cd(s) + Fe3+(aq) Fe2+(aq) + Cd2+(aq)
B) Cd(s) + 2 Fe3+(aq) 2 Fe2+(aq) + Cd2+(aq)
C) 2 Cd(s) + Fe3+(aq) Fe2+(aq) + 2 Cd2+(aq)
D) 2 Cd(s) + Fe3+(aq) 2 Fe2+(aq) + Cd2+(aq)
A) Cd(s) + Fe3+(aq) Fe2+(aq) + Cd2+(aq)
B) Cd(s) + 2 Fe3+(aq) 2 Fe2+(aq) + Cd2+(aq)
C) 2 Cd(s) + Fe3+(aq) Fe2+(aq) + 2 Cd2+(aq)
D) 2 Cd(s) + Fe3+(aq) 2 Fe2+(aq) + Cd2+(aq)

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
11
Which statement is correct?
A) all electrolytic cells are voltaic cells
B) all voltaic cells are electrolytic cells
C) metals corrode by an electrolytic process
D) voltaic cells and electrolytic cells are both electrochemical cells
E) all electrochemical cells are electrolytic cells
A) all electrolytic cells are voltaic cells
B) all voltaic cells are electrolytic cells
C) metals corrode by an electrolytic process
D) voltaic cells and electrolytic cells are both electrochemical cells
E) all electrochemical cells are electrolytic cells

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
12
Which compound contains the atom with the highest oxidation number?
A) FeCl3
B) SnH4
C) NaClO4
D) CrO3
E) H2O2
A) FeCl3
B) SnH4
C) NaClO4
D) CrO3
E) H2O2

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
13
Which is not true of the standard conditions for electrochemical cell measurements?
A) the cell voltage is always positive
B) solutes are at 1.0 M concentration
C) solids are in the pure state
D) liquids are in the pure state
E) gases are at 1 bar pressure
A) the cell voltage is always positive
B) solutes are at 1.0 M concentration
C) solids are in the pure state
D) liquids are in the pure state
E) gases are at 1 bar pressure

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
14
Which change describes an oxidation half-reaction?
A) decrease in oxidation number
B) loss of electrons
C) electrons as reactants
D) reactant acting as an oxidizing agent
E) pure oxygen becoming oxide ion
A) decrease in oxidation number
B) loss of electrons
C) electrons as reactants
D) reactant acting as an oxidizing agent
E) pure oxygen becoming oxide ion

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
15
In the reaction shown below, ____ is the oxidizing agent and ____ the reducing agent. 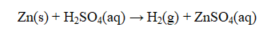
A) Zn2+; H2
B) Zn; H+
C) H2; Zn2+
D) H+; Zn2+
E) H+; Zn
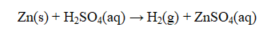
A) Zn2+; H2
B) Zn; H+
C) H2; Zn2+
D) H+; Zn2+
E) H+; Zn

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
16
The study of the relationships between electron flow and redox reactions is called
A) nuclear chemistry.
B) electrochemistry.
C) thermodynamics.
D) electrodynamics.
E) inorganic chemistry.
A) nuclear chemistry.
B) electrochemistry.
C) thermodynamics.
D) electrodynamics.
E) inorganic chemistry.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
17
Which of these components must be present in a redox reaction?
A) a substance present as an element which becomes incorporated into a molecule, or vice versa
B) an atom whose oxidation number increases
C) an atom whose oxidation number decreases
D) either b or c
E) both b and c
A) a substance present as an element which becomes incorporated into a molecule, or vice versa
B) an atom whose oxidation number increases
C) an atom whose oxidation number decreases
D) either b or c
E) both b and c

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
18
A voltaic cell is one in which
A) the standard reduction potentials of the two half-cell reactions are equal to one another
B) the standard cell voltage is positive
C) the standard cell voltage is negative
D) both a and b are correct
E) both a and c are correct
A) the standard reduction potentials of the two half-cell reactions are equal to one another
B) the standard cell voltage is positive
C) the standard cell voltage is negative
D) both a and b are correct
E) both a and c are correct

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 18-2 Use this list of half-reactions to answer the following question(s).
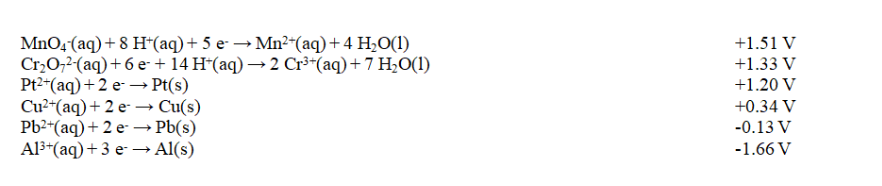
Refer to Exhibit 18-2. How many different cells with E cell in excess of +1.90 V can be constructed using the half-reactions given in the table?
A) 1
B) 2
C) 3
D) 4
E) 5
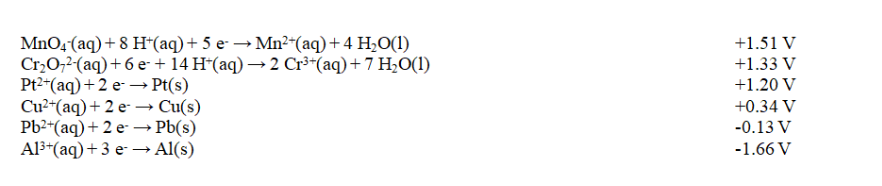
Refer to Exhibit 18-2. How many different cells with E cell in excess of +1.90 V can be constructed using the half-reactions given in the table?
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
20
When the reaction shown below is balanced, the coefficients are ____, and ____ electrons are transferred. 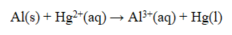
A) 1, 3, 1, and 3; 6
B) 2, 3, 2, and 3; 6
C) 3, 2, 3, and 2; 6
D) 2, 3, 2, and 3; 12
E) 3, 2, 3, and 2; 12
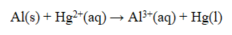
A) 1, 3, 1, and 3; 6
B) 2, 3, 2, and 3; 6
C) 3, 2, 3, and 2; 6
D) 2, 3, 2, and 3; 12
E) 3, 2, 3, and 2; 12

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
21
The value of E cell for the cell shown below is + 1.41 V. 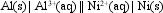
What is the value of Ecell at 25 C if the concentration of Al3+(aq) is 0.050 M, and of Ni2+(aq), 2.0 M?
A) +1.34 V
B) +1.38 V
C) +1.41 V
D) +1.44 V
E) +1.48 V
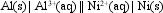
What is the value of Ecell at 25 C if the concentration of Al3+(aq) is 0.050 M, and of Ni2+(aq), 2.0 M?
A) +1.34 V
B) +1.38 V
C) +1.41 V
D) +1.44 V
E) +1.48 V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
22
The value of E cell for an aluminum-nickel electrochemical cell is +1.41 V at 25 C. The value of K for this cell under standard conditions is
A) <<0.01
B) » 0.01
C) » 1
D) » 100
E) >>100
A) <<0.01
B) » 0.01
C) » 1
D) » 100
E) >>100

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
23
Exhibit 18-2 Use this list of half-reactions to answer the following question(s).
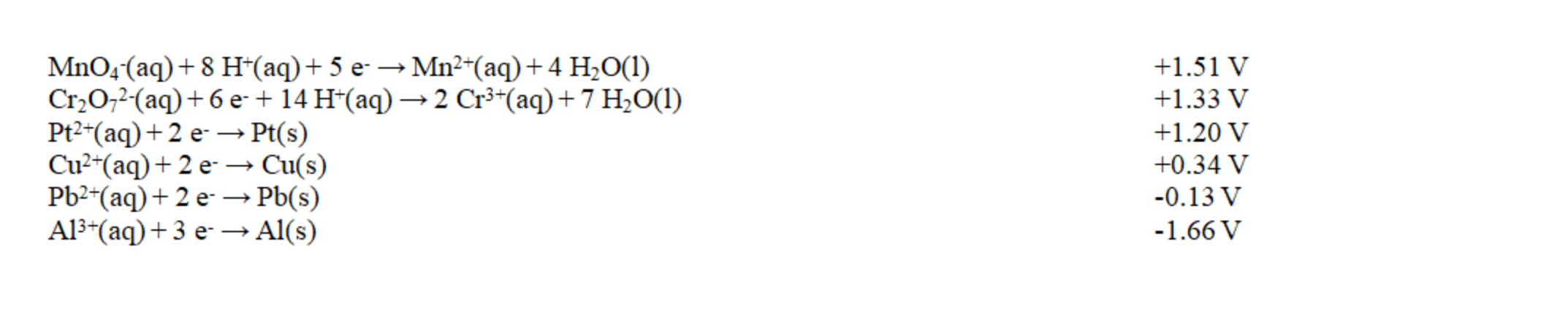 Refer to Exhibit 18-2. The strongest reducing agent in the table is
Refer to Exhibit 18-2. The strongest reducing agent in the table is
A) Al(s).
B) Al3+(aq).
C) H+(aq).
D) MnO4-(aq).
E) Mn2+(aq).
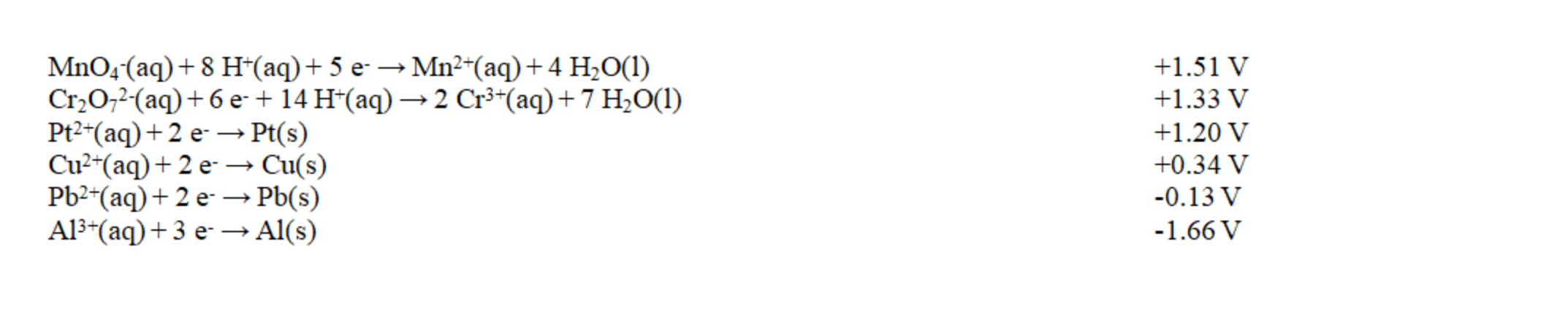 Refer to Exhibit 18-2. The strongest reducing agent in the table is
Refer to Exhibit 18-2. The strongest reducing agent in the table isA) Al(s).
B) Al3+(aq).
C) H+(aq).
D) MnO4-(aq).
E) Mn2+(aq).

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
24
The value of E cell for an aluminum-nickel electrochemical cell is +1.41 V at 25 C. Calculate the value of DG for this cell under standard conditions.
A) -816 kJ
B) -680 kJ
C) -272 kJ
D) +403 kJ
E) +680 kJ
A) -816 kJ
B) -680 kJ
C) -272 kJ
D) +403 kJ
E) +680 kJ

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
25
An electrolytic reaction is a system in which
A) the reaction conditions are manipulated to change the value of E cell to a favorable one.
B) a reactant-favored reaction is forced to produce electricity by the input of heat or light.
C) the same element is both oxidized and reduced.
D) electricity is used to produce a chemical reaction.
E) a chemical reaction is used to produce electricity.
A) the reaction conditions are manipulated to change the value of E cell to a favorable one.
B) a reactant-favored reaction is forced to produce electricity by the input of heat or light.
C) the same element is both oxidized and reduced.
D) electricity is used to produce a chemical reaction.
E) a chemical reaction is used to produce electricity.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the value of Ecell for a zinc-platinum electrochemical cell when the concentration of Pt2+(aq) is 0.050 M and the concentration of Zn2+(aq) is 1.1 M. E cell = 1.96 V under standard conditions. Pt is the less active metal.
A) 2.04 V
B) 2.00 V
C) 1.96 V
D) 1.92 V
E) 1.88 V
A) 2.04 V
B) 2.00 V
C) 1.96 V
D) 1.92 V
E) 1.88 V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
27
Exhibit 18-2 Use this list of half-reactions to answer the following question(s).
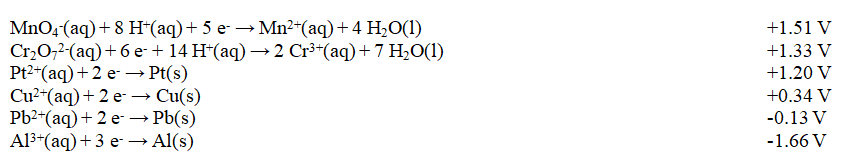 Refer to Exhibit 18-2. The strongest oxidizing agent in the table is
Refer to Exhibit 18-2. The strongest oxidizing agent in the table is
A) Al(s)
B) Al3+(aq)
C) H+(aq)
D) MnO4-(aq)
E) Mn2+(aq)
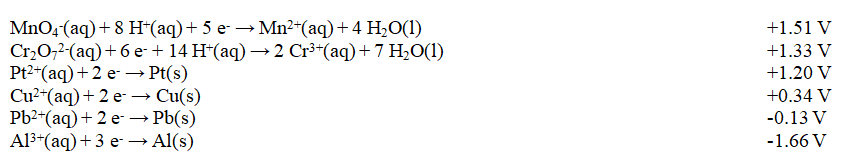 Refer to Exhibit 18-2. The strongest oxidizing agent in the table is
Refer to Exhibit 18-2. The strongest oxidizing agent in the table isA) Al(s)
B) Al3+(aq)
C) H+(aq)
D) MnO4-(aq)
E) Mn2+(aq)

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
28
A mass of 0.839 g of a divalent metal is plated out of a solution of the divalent metal ion. This takes 67.2 min at a current of 0.63 A. What is the metal? (Hint: find its atomic mass.)
A) Cd
B) Cu
C) Hg
D) Fe
E) Mg
A) Cd
B) Cu
C) Hg
D) Fe
E) Mg

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the cell reaction 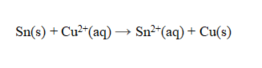 The value of E cell is 0.447 V at 25 C. Calculate the value of DG and K for this cell.
The value of E cell is 0.447 V at 25 C. Calculate the value of DG and K for this cell.
A) -86.3 kJ; 1.26 × 1015
B) -43.1 kJ; 1.37 × 1043
C) 43.1 kJ; 3.55 × 107
D) 86.3 kJ; 7.92 × 10-16
E) 86.3 kJ; 2.00 × 1086
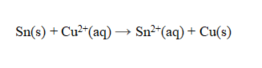 The value of E cell is 0.447 V at 25 C. Calculate the value of DG and K for this cell.
The value of E cell is 0.447 V at 25 C. Calculate the value of DG and K for this cell.A) -86.3 kJ; 1.26 × 1015
B) -43.1 kJ; 1.37 × 1043
C) 43.1 kJ; 3.55 × 107
D) 86.3 kJ; 7.92 × 10-16
E) 86.3 kJ; 2.00 × 1086

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
30
Which process could not be an electrolytic reaction?
A) 2 H2O(l) 2 H2(g) + O2(g)
B) 2 Al2O3(l) 4 Al(l) + 3 O2(g)
C) Na+(aq) + Cl-(aq) 2 Na(l) + Cl2(g)
D) 2 Fe(s) + 3 O2(g) Fe2O3(s)
E) 2 PbSO4(s) + 4 H2O(l) Pb(s) + PbO2(s) + 2 H2SO4(aq)
A) 2 H2O(l) 2 H2(g) + O2(g)
B) 2 Al2O3(l) 4 Al(l) + 3 O2(g)
C) Na+(aq) + Cl-(aq) 2 Na(l) + Cl2(g)
D) 2 Fe(s) + 3 O2(g) Fe2O3(s)
E) 2 PbSO4(s) + 4 H2O(l) Pb(s) + PbO2(s) + 2 H2SO4(aq)

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
31
Which statement about lead storage batteries is not correct?
A) The value of E cell is slightly more than 2 V
B) The anode reaction involves the conversion of Pb to Pb2+
C) Nitric acid serves as the electrolyte
D) The cathode reaction involves the conversion of Pb4+ to Pb2+
E) In order to provide 12 volts, the battery consists of six cells
A) The value of E cell is slightly more than 2 V
B) The anode reaction involves the conversion of Pb to Pb2+
C) Nitric acid serves as the electrolyte
D) The cathode reaction involves the conversion of Pb4+ to Pb2+
E) In order to provide 12 volts, the battery consists of six cells

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
32
Exhibit 18-2 Use this list of half-reactions to answer the following question(s).
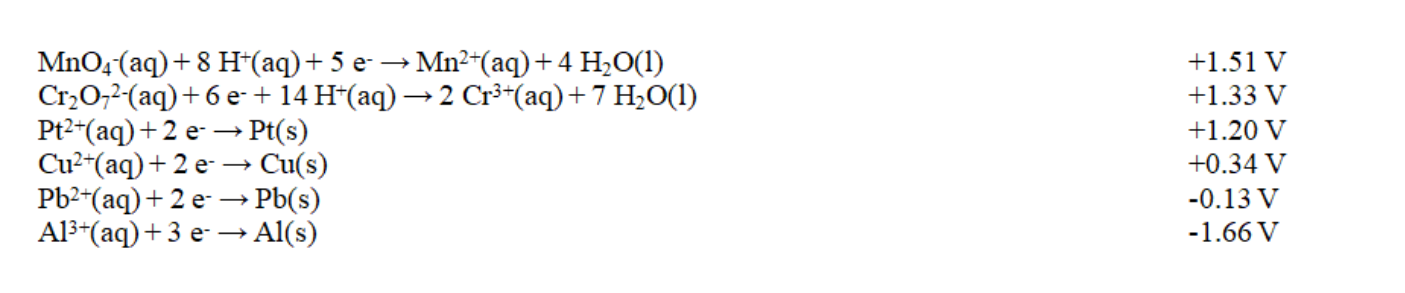 Refer to Exhibit 18-2. The potential for the product-favored reaction involving aluminum and copper metals, Al3+(aq) and Cu2+(aq), is
Refer to Exhibit 18-2. The potential for the product-favored reaction involving aluminum and copper metals, Al3+(aq) and Cu2+(aq), is
A) 2.17 V.
B) 2.00 V.
C) 1.79 V.
D) 1.32 V.
E) 1.15 V.
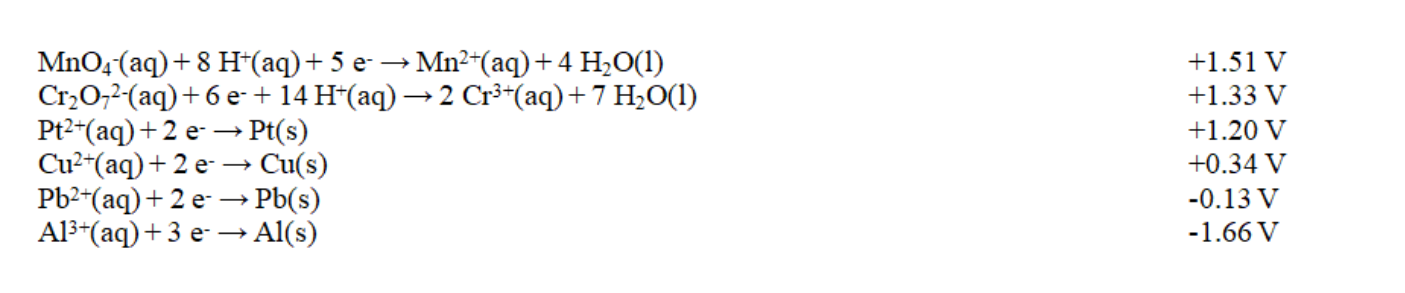 Refer to Exhibit 18-2. The potential for the product-favored reaction involving aluminum and copper metals, Al3+(aq) and Cu2+(aq), is
Refer to Exhibit 18-2. The potential for the product-favored reaction involving aluminum and copper metals, Al3+(aq) and Cu2+(aq), isA) 2.17 V.
B) 2.00 V.
C) 1.79 V.
D) 1.32 V.
E) 1.15 V.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
33
Which statement concerning the proton exchange membrane fuel cell is not correct?
A) At the anode, hydrogen gas is reduced to form water.
B) At the cathode, oxygen is reduced to water.
C) The cell uses platinum as a catalyst.
D) The actual fuel cell reaction causes no pollution of any kind.
E) A limiting factor is the cost of producing hydrogen as the fuel.
A) At the anode, hydrogen gas is reduced to form water.
B) At the cathode, oxygen is reduced to water.
C) The cell uses platinum as a catalyst.
D) The actual fuel cell reaction causes no pollution of any kind.
E) A limiting factor is the cost of producing hydrogen as the fuel.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following would require the most Faradays of electricity?
A) converting 2.0 mol of Al3+ into Al
B) converting 2.0 mol of Cr3+ into Cr
C) converting 1.5 mol of Cr6+ into Cr
D) converting 3.0 mol of Sn4+ into Sn2+
E) converting 2.0 mol of Sn4+ into Sn
A) converting 2.0 mol of Al3+ into Al
B) converting 2.0 mol of Cr3+ into Cr
C) converting 1.5 mol of Cr6+ into Cr
D) converting 3.0 mol of Sn4+ into Sn2+
E) converting 2.0 mol of Sn4+ into Sn

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
35
A fuel cell is
A) an electrolytic cell that is used to supply electrical power
B) an electrochemical cell that converts chemical energy directly into electricity
C) a cell in which a hydrogen-oxygen is ignited to produced electricity
D) typically not susceptible to contamination
E) currently both technically feasible and commercially viable as a source of electrical power
A) an electrolytic cell that is used to supply electrical power
B) an electrochemical cell that converts chemical energy directly into electricity
C) a cell in which a hydrogen-oxygen is ignited to produced electricity
D) typically not susceptible to contamination
E) currently both technically feasible and commercially viable as a source of electrical power

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
36
The relationship between Gibbs free energy and E cell is DG =
A)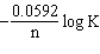
B)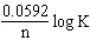
C) -nFE cell.
D) nFE cell.
E) -RT ln K.
A)
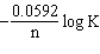
B)
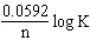
C) -nFE cell.
D) nFE cell.
E) -RT ln K.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
37
Exhibit 18-2 Use this list of half-reactions to answer the following question(s).
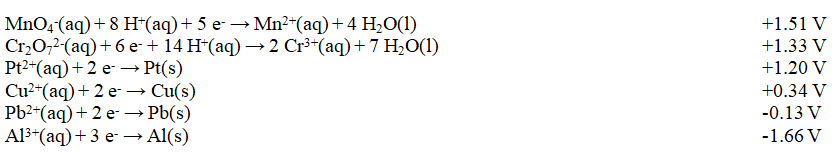 Refer to Exhibit 18-2. Which of these combinations would result in a spontaneous reaction?
Refer to Exhibit 18-2. Which of these combinations would result in a spontaneous reaction?
A) Al3+(aq) and Cr3+(aq)
B) Al3+(aq) and Cu(s)
C) Cr2O72-(aq) and MnO4-(aq)
D) Cu(s) and MnO4-(aq)
E) Pt(s) and Pb2+(aq)
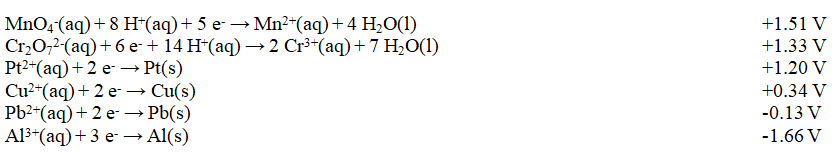 Refer to Exhibit 18-2. Which of these combinations would result in a spontaneous reaction?
Refer to Exhibit 18-2. Which of these combinations would result in a spontaneous reaction?A) Al3+(aq) and Cr3+(aq)
B) Al3+(aq) and Cu(s)
C) Cr2O72-(aq) and MnO4-(aq)
D) Cu(s) and MnO4-(aq)
E) Pt(s) and Pb2+(aq)

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
38
The value of Ecell at 25 C for the cell shown below is +1.27 V. What is the value of E cell? 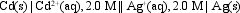
A) 1.57 V
B) 1.28 V
C) 1.26 V
D) 1.23 V
E) 0.97 V
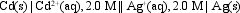
A) 1.57 V
B) 1.28 V
C) 1.26 V
D) 1.23 V
E) 0.97 V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
39
A secondary cell is one which ____; this is possible because ____.
A) is driven by a primary cell; the cell reactions are coupled
B) is disposable without harming the environment; no mercury is used
C) is rechargeable; the cell reaction is reversible
D) has double the lifetime of a primary cell; two primary cells are placed in parallel
E) has double the voltage of a primary cell; two primary cells are placed in series
A) is driven by a primary cell; the cell reactions are coupled
B) is disposable without harming the environment; no mercury is used
C) is rechargeable; the cell reaction is reversible
D) has double the lifetime of a primary cell; two primary cells are placed in parallel
E) has double the voltage of a primary cell; two primary cells are placed in series

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
40
A primary battery is one in which
A) the electrochemical reactions can be easily reversed
B) the electrochemical reactions cannot be easily reversed
C) the battery is "dead" and it must be discarded
D) the cell is rechargeable
E) both b and c are correct
A) the electrochemical reactions can be easily reversed
B) the electrochemical reactions cannot be easily reversed
C) the battery is "dead" and it must be discarded
D) the cell is rechargeable
E) both b and c are correct

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
41
Is the part of a flashlight battery that is marked "+" the anode or the cathode? Explain why it is labeled in that way.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
42
Explain why galvanizing steel chain-link fencing is a more effective strategy to prevent corrosion than simply painting it.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
43
Galvanizing a metal (coating its surface with zinc) to prevent corrosion works because
A) the coating limits access of oxygen to the metal surface
B) the coating reacts to give an impermeable layer of zinc hydroxide
C) the zinc coating provides cathodic protection
D) of reasons a and b.
E) of reasons a, b, and c.
A) the coating limits access of oxygen to the metal surface
B) the coating reacts to give an impermeable layer of zinc hydroxide
C) the zinc coating provides cathodic protection
D) of reasons a and b.
E) of reasons a, b, and c.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
44
Calculate the time needed to plate out 175 g of nickel from a Ni2+ solution when a current of 10.0 A is applied.
A) 4.00 hr
B) 8.00 hr
C) 9.40 hr
D) 16.0 hr
E) 32.0 hr
A) 4.00 hr
B) 8.00 hr
C) 9.40 hr
D) 16.0 hr
E) 32.0 hr

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
45
Refer to the following values of standard reduction potentials.
 a. Write the balanced overall reaction for gold reacting with zinc(II) ion.
a. Write the balanced overall reaction for gold reacting with zinc(II) ion.
b. Calculate the value of E°cell for the reaction.
c. How many electrons are transferred in this reaction? Explain.
d. Calculate the value of DG° for this cell at 25°C.
e. Is this reaction product-favored or reactant-favored? Explain how your answers in Parts b, d, and e support this conclusion.
 a. Write the balanced overall reaction for gold reacting with zinc(II) ion.
a. Write the balanced overall reaction for gold reacting with zinc(II) ion.b. Calculate the value of E°cell for the reaction.
c. How many electrons are transferred in this reaction? Explain.
d. Calculate the value of DG° for this cell at 25°C.
e. Is this reaction product-favored or reactant-favored? Explain how your answers in Parts b, d, and e support this conclusion.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
46
Calculate the mass of cobalt that will be deposited when a current of 2.00 A is passed through a solution of CoSO4 for 10.0 hours.
A) 6.11 × 10-3 g
B) 0.366 g
C) 4.40 g
D) 8.72 g
E) 22.0 g
A) 6.11 × 10-3 g
B) 0.366 g
C) 4.40 g
D) 8.72 g
E) 22.0 g

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
47
Unwanted oxidation of a metal exposed to the environment is
A) galvanization.
B) electroplating.
C) electrolysis.
D) corrosion.
E) cathodic protection.
A) galvanization.
B) electroplating.
C) electrolysis.
D) corrosion.
E) cathodic protection.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
48
Which statement concerning the number of electrons involved in electrolysis is not correct?
A) The total charge is obtained by multiplying the current in amps by the time in seconds.
B) The charge associated with one mole of electrons is called the Faraday.
C) In producing one mole of Cl2(g) from Cl-(aq), one mole of electrons is produced.
D) When one mole of Fe(s) is produced from Fe3+(aq), three moles of electrons are needed.
E) Electroplating of one mole of silver from a solution of silver ions requires one mole of electrons.
A) The total charge is obtained by multiplying the current in amps by the time in seconds.
B) The charge associated with one mole of electrons is called the Faraday.
C) In producing one mole of Cl2(g) from Cl-(aq), one mole of electrons is produced.
D) When one mole of Fe(s) is produced from Fe3+(aq), three moles of electrons are needed.
E) Electroplating of one mole of silver from a solution of silver ions requires one mole of electrons.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
49
Although aluminum is a moderately reactive metal, it can be regarded as unreactive in everyday use because a layer of aluminum oxide coats the surface and prevents further oxidation. This is an example of
A) anodic inhibition.
B) cathodic inhibition.
C) galvanization.
D) cathodic protection.
E) anodic protection.
A) anodic inhibition.
B) cathodic inhibition.
C) galvanization.
D) cathodic protection.
E) anodic protection.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
50
Explain, with reference to the Nernst equation, how it is possible for a cell to have a positive cell potential under standard conditions, but a negative cell potential under other conditions.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
51
Answer the following questions:
a. Briefly explain why aluminum is usually isolated from its ore by electrolysis, not by chemical reduction.
b. Explain why this electrolysis has to be performed on a molten aluminum salt, not in aqueous solution.
a. Briefly explain why aluminum is usually isolated from its ore by electrolysis, not by chemical reduction.
b. Explain why this electrolysis has to be performed on a molten aluminum salt, not in aqueous solution.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
52
If all of the following metals are part of a metal piping system, and an electrolyte solution is pumped through the system, which metal will corrode the most?
A) Cu (E anode = +0.34 V)
B) Fe (E anode = -0.44 V)
C) Sn (E anode = -0.14 V)
D) Mg (E anode = -2.37 V)
E) Ni (E anode = -0.25 V)
A) Cu (E anode = +0.34 V)
B) Fe (E anode = -0.44 V)
C) Sn (E anode = -0.14 V)
D) Mg (E anode = -2.37 V)
E) Ni (E anode = -0.25 V)

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
53
An underground steel fuel pipe buried in an area that is infiltrated by sea water could be protected against corrosion by using
A) anodic inhibition
B) cathodic protection
C) both anodic inhibition and cathodic protection
D) any of these methods would be effective
E) none of these methods would be effective
A) anodic inhibition
B) cathodic protection
C) both anodic inhibition and cathodic protection
D) any of these methods would be effective
E) none of these methods would be effective

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
54
Balance the following redox equation.
 The oxidation process is clearly
The oxidation process is clearly
 The oxidation process is clearly
The oxidation process is clearly
Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck


