Exam 18: Electrochemistry and Its Applications
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
Which is not true of the standard conditions for electrochemical cell measurements?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
A
A mass of 0.839 g of a divalent metal is plated out of a solution of the divalent metal ion. This takes 67.2 min at a current of 0.63 A. What is the metal? (Hint: find its atomic mass.)
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
B
Exhibit 18-2 Use this list of half-reactions to answer the following question(s).
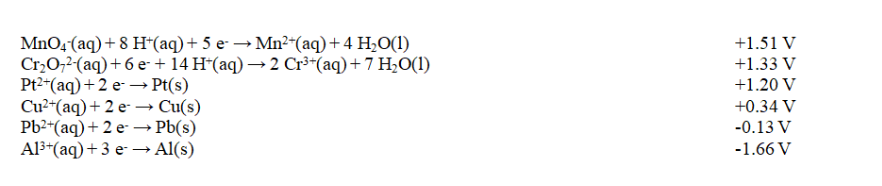 Refer to Exhibit 18-2. How many different cells with E cell in excess of +1.90 V can be constructed using the half-reactions given in the table?
Refer to Exhibit 18-2. How many different cells with E cell in excess of +1.90 V can be constructed using the half-reactions given in the table?
(Multiple Choice)
4.8/5  (32)
(32)
Answer the following questions:
a. Briefly explain why aluminum is usually isolated from its ore by electrolysis, not by chemical reduction.
b. Explain why this electrolysis has to be performed on a molten aluminum salt, not in aqueous solution.
(Essay)
4.7/5  (36)
(36)
Which statement concerning the number of electrons involved in electrolysis is not correct?
(Multiple Choice)
4.8/5  (33)
(33)
Explain why galvanizing steel chain-link fencing is a more effective strategy to prevent corrosion than simply painting it.
(Essay)
4.9/5  (38)
(38)
Exhibit 18-2 Use this list of half-reactions to answer the following question(s).
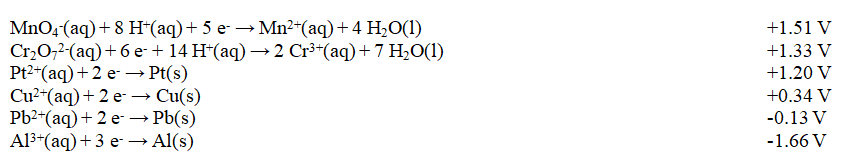 Refer to Exhibit 18-2. The strongest oxidizing agent in the table is
Refer to Exhibit 18-2. The strongest oxidizing agent in the table is
(Multiple Choice)
4.9/5  (34)
(34)
Select the material containing the element with the highest oxidation number and determine how many electrons are required to reduce that element to an oxidation number of zero. 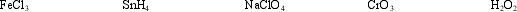
(Multiple Choice)
4.8/5  (43)
(43)
Which of these components must be present in a redox reaction?
(Multiple Choice)
4.8/5  (38)
(38)
An underground steel fuel pipe buried in an area that is infiltrated by sea water could be protected against corrosion by using
(Multiple Choice)
4.8/5  (33)
(33)
The value of E cell for the cell shown below is + 1.41 V. 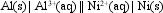 What is the value of Ecell at 25 C if the concentration of Al3+(aq) is 0.050 M, and of Ni2+(aq), 2.0 M?
What is the value of Ecell at 25 C if the concentration of Al3+(aq) is 0.050 M, and of Ni2+(aq), 2.0 M?
(Multiple Choice)
4.8/5  (28)
(28)
The value of Ecell at 25 C for the cell shown below is +1.27 V. What is the value of E cell? 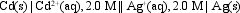
(Multiple Choice)
4.8/5  (32)
(32)
Refer to the following values of standard reduction potentials.
 a. Write the balanced overall reaction for gold reacting with zinc(II) ion.
b. Calculate the value of E°cell for the reaction.
c. How many electrons are transferred in this reaction? Explain.
d. Calculate the value of DG° for this cell at 25°C.
e. Is this reaction product-favored or reactant-favored? Explain how your answers in Parts b, d, and e support this conclusion.
a. Write the balanced overall reaction for gold reacting with zinc(II) ion.
b. Calculate the value of E°cell for the reaction.
c. How many electrons are transferred in this reaction? Explain.
d. Calculate the value of DG° for this cell at 25°C.
e. Is this reaction product-favored or reactant-favored? Explain how your answers in Parts b, d, and e support this conclusion.
(Essay)
4.8/5  (38)
(38)
Which statement concerning the proton exchange membrane fuel cell is not correct?
(Multiple Choice)
4.9/5  (46)
(46)
If cadmium metal and the Fe(III) ion are mixed in aqueous solution, a solution containing Cd(II) and Fe(II) results. The balanced equation for this process is
(Multiple Choice)
4.8/5  (44)
(44)
Showing 1 - 20 of 54
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)