Deck 17: Thermodynamics: Directionality of Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/56
Play
Full screen (f)
Deck 17: Thermodynamics: Directionality of Chemical Reactions
1
Five coins are tossed. Which combination of heads (H) and tails (T) is least likely?
A) THTHT
B) TTTHT
C) HTTHT
D) HHTHT
E) THHTT
A) THTHT
B) TTTHT
C) HTTHT
D) HHTHT
E) THHTT
TTTHT
2
Which statement is correct?
A) A product-favored process requires a continuous input of energy to continue
B) A product-favored process may start on its own without much initial energy input
C) A product-favored process, when run in reverse, is also product-favored
D) Once started, a reactant-favored process will continue on its own
E) The term non-spontaneous is sometimes used to describe a product-favored process
A) A product-favored process requires a continuous input of energy to continue
B) A product-favored process may start on its own without much initial energy input
C) A product-favored process, when run in reverse, is also product-favored
D) Once started, a reactant-favored process will continue on its own
E) The term non-spontaneous is sometimes used to describe a product-favored process
A product-favored process may start on its own without much initial energy input
3
Nature favors exothermic reactions because after such a reaction
A) the energy previously concentrated in a few particles is now dispersed over more particles in the system.
B) the energy previously concentrated in a few particles is now dispersed over more particles in the surroundings.
C) the energy previously concentrated in a few particles is now dispersed over more particles in both the system and the surroundings.
D) the energy previously held in many particles is now concentrated in a few, resulting in a temperature rise.
E) the energy previously held in many particles is now concentrated in a few, resulting in a temperature fall.
A) the energy previously concentrated in a few particles is now dispersed over more particles in the system.
B) the energy previously concentrated in a few particles is now dispersed over more particles in the surroundings.
C) the energy previously concentrated in a few particles is now dispersed over more particles in both the system and the surroundings.
D) the energy previously held in many particles is now concentrated in a few, resulting in a temperature rise.
E) the energy previously held in many particles is now concentrated in a few, resulting in a temperature fall.
the energy previously concentrated in a few particles is now dispersed over more particles in both the system and the surroundings.
4
Which statement is false?
A) entropy is a measurement of the nanoscale dispersal of energy in a sample of matter.
B) entropies of ionic solids with similar formulas are larger when the attractions among the ions are stronger
C) A perfect crystal of any substance at 0 K has minimum entropy.
D) The standard molar entropy of a substance at temperature T is a measure of the quantity of energy that must be dispersed in that substance for it to exist at T.
E) Temperatures of a few nanokelvins can be achieved in a Bose-Einstein condensate.
A) entropy is a measurement of the nanoscale dispersal of energy in a sample of matter.
B) entropies of ionic solids with similar formulas are larger when the attractions among the ions are stronger
C) A perfect crystal of any substance at 0 K has minimum entropy.
D) The standard molar entropy of a substance at temperature T is a measure of the quantity of energy that must be dispersed in that substance for it to exist at T.
E) Temperatures of a few nanokelvins can be achieved in a Bose-Einstein condensate.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
5
Which has the highest entropy at a given temperature?
A) SO2(s)
B) S(s)
C) O2(g)
D) SO2(l)
E) SO2(g)
A) SO2(s)
B) S(s)
C) O2(g)
D) SO2(l)
E) SO2(g)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
6
Two substances, A and B, have identical enthalpies of vaporization. They boil at 164 C and 236 C, respectively. If the entropy of vaporization of A is 87.2 J/K, what is the entropy of vaporization of B, in J/K?
A) 102
B) 60.6
C) 126
D) 38.3
E) 74.9
A) 102
B) 60.6
C) 126
D) 38.3
E) 74.9

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
7
At a particular temperature, a reactant-favored process is one in which
A) reactants rapidly react with one another to form products
B) products rapidly react with one another to form reactants
C) products predominate over reactants
D) reactants predominate over products
E) large amounts of energy are produced
A) reactants rapidly react with one another to form products
B) products rapidly react with one another to form reactants
C) products predominate over reactants
D) reactants predominate over products
E) large amounts of energy are produced

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement is false?
A) entropies of gases are usually much larger that those of liquids, which in turn are usually larger than those of solids
B) entropy usually increases when a gas is dissolved in a liquid
C) entropies of more complex molecules are larger than those of simpler molecules
D) entropy usually increases when a pure liquid or solid dissolves in a solvent
E) entropies of ionic solids that have similar formulas are smaller when the attractions among the ions are stronger
A) entropies of gases are usually much larger that those of liquids, which in turn are usually larger than those of solids
B) entropy usually increases when a gas is dissolved in a liquid
C) entropies of more complex molecules are larger than those of simpler molecules
D) entropy usually increases when a pure liquid or solid dissolves in a solvent
E) entropies of ionic solids that have similar formulas are smaller when the attractions among the ions are stronger

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
9
Which statement is false?
A) energy will disperse unless it is hindered from doing so
B) energy will not disperse unless it is forced to do so
C) energy has a higher probability of being spread out over many molecules than of being concentrated in a few
D) energy is dispersed when a system of atoms or molecules expands to occupy a larger volume
E) gases expanding into larger volumes illustrate energy dispersal
A) energy will disperse unless it is hindered from doing so
B) energy will not disperse unless it is forced to do so
C) energy has a higher probability of being spread out over many molecules than of being concentrated in a few
D) energy is dispersed when a system of atoms or molecules expands to occupy a larger volume
E) gases expanding into larger volumes illustrate energy dispersal

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
10
How many of the following processes could be described as product-favored: dissolving the first spoonful of sugar in a cup of hot coffee; alcohol separating from a beer sample at 5 C; dissolving the ninth spoonful of sugar in a cup of hot coffee; sediment settling out of an muddy water sample?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the options given for the distribution of three units of energy to three particles. Which arrangement is least likely?
A) A*B*C*
B) A**B*
C) A**C*
D) A***
E) B**C*
A) A*B*C*
B) A**B*
C) A**C*
D) A***
E) B**C*

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
12
DS for a reaction can be calculated from the following equation.
A) DS = (Smoles product) ×S product - (Smoles reactant) ×S reactant
B) DS = S(moles product × S product) - S(moles reactant × S reactant)
C) DS = S(moles reactant × S reactant) - S(moles product × S product)
D) DS = S(moles product × S reactant) - S(moles reactant × S product)
E) DS = S(moles product × S product) + S(moles reactant × S reactant)
A) DS = (Smoles product) ×S product - (Smoles reactant) ×S reactant
B) DS = S(moles product × S product) - S(moles reactant × S reactant)
C) DS = S(moles reactant × S reactant) - S(moles product × S product)
D) DS = S(moles product × S reactant) - S(moles reactant × S product)
E) DS = S(moles product × S product) + S(moles reactant × S reactant)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
13
How many of the following processes involve a decrease in disorder: humidifying dry air; distilling crude oil into gasoline, fuel oil, and jet fuel; filtering solid impurities out of a mixture; adding sand to water?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
14
Which has the lowest entropy at a given temperature?
A) H2(g)
B) O2(g)
C) H2O(g)
D) H2O(l)
E) H2O(s)
A) H2(g)
B) O2(g)
C) H2O(g)
D) H2O(l)
E) H2O(s)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
15
Which process involves a decrease of entropy for the system?
A) mixing aqueous solutions of NaCl and KNO3 together
B) spreading grass seed on a lawn
C) raking and bagging leaves in the fall
D) shuffling a deck of cards
E) raking and burning leaves in the fall
A) mixing aqueous solutions of NaCl and KNO3 together
B) spreading grass seed on a lawn
C) raking and bagging leaves in the fall
D) shuffling a deck of cards
E) raking and burning leaves in the fall

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
16
Which has the highest entropy?
A) H2O(g) at 150 C
B) H2O(g) at 100 C
C) H2O(l) at 100 C
D) H2O(l) at 4 C (the temperature of maximum density)
E) H2O(s) at -50 C
A) H2O(g) at 150 C
B) H2O(g) at 100 C
C) H2O(l) at 100 C
D) H2O(l) at 4 C (the temperature of maximum density)
E) H2O(s) at -50 C

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
17
The boiling point of tin is 232 C. The heat of vaporization of tin at its boiling point is 247 kJ. The entropy of vaporization is
A) 2045 J/K.
B) 1065 J/K.
C) 939 J/K.
D) 489 J/K.
E) 2.04 J/K.
A) 2045 J/K.
B) 1065 J/K.
C) 939 J/K.
D) 489 J/K.
E) 2.04 J/K.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
18
Which statement is correct?
A) The entropy of a pure substance is greater in the liquid state than in the gaseous state.
B) The entropy of a pure substance in a given state decreases as the temperature rises.
C) Because of the larger charges, the entropy of CaO(s) is greater than the entropy of KF(s).
D) Entropy decreases when ethanol, C2H5OH, dissolves in water.
E) A bottle of carbonated water has lower entropy than the same masses of carbon dioxide gas and distilled water.
A) The entropy of a pure substance is greater in the liquid state than in the gaseous state.
B) The entropy of a pure substance in a given state decreases as the temperature rises.
C) Because of the larger charges, the entropy of CaO(s) is greater than the entropy of KF(s).
D) Entropy decreases when ethanol, C2H5OH, dissolves in water.
E) A bottle of carbonated water has lower entropy than the same masses of carbon dioxide gas and distilled water.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
19
Compression of a gas is
A) reactant-favored, since energy in the compressed gas is more dispersed.
B) reactant-favored, since matter in the compressed gas is more dispersed.
C) reactant-favored, since energy in the compressed gas is less dispersed.
D) product-favored, since energy in the compressed gas is less dispersed.
E) product-favored, since energy in the compressed gas is more dispersed.
A) reactant-favored, since energy in the compressed gas is more dispersed.
B) reactant-favored, since matter in the compressed gas is more dispersed.
C) reactant-favored, since energy in the compressed gas is less dispersed.
D) product-favored, since energy in the compressed gas is less dispersed.
E) product-favored, since energy in the compressed gas is more dispersed.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
20
Which process is reactant-favored?
A) decomposition of iron ore into pure iron
B) the melting of ice at 25 C
C) diffusion of the odor of cooking food
D) the freezing of water at -5 C
E) dissociation of a strong acid in water
A) decomposition of iron ore into pure iron
B) the melting of ice at 25 C
C) diffusion of the odor of cooking food
D) the freezing of water at -5 C
E) dissociation of a strong acid in water

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
21
A reaction cannot change between being product-favored and being reactant-favored when
A) DH rxn < 0 and DS rxn > 0
B) DH rxn < 0 and DS rxn < 0
C) DH rxn > 0 and DS rxn < 0
D) DH rxn > 0 and DS rxn > 0
E) DH rxn = 0 and DS rxn < 0
A) DH rxn < 0 and DS rxn > 0
B) DH rxn < 0 and DS rxn < 0
C) DH rxn > 0 and DS rxn < 0
D) DH rxn > 0 and DS rxn > 0
E) DH rxn = 0 and DS rxn < 0

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
22
A certain reaction has DH rxn = +177.8 kJ, and DS rxn = +160.5 J/K. Above what temperature does it become product-favored ?
A) 384 C
B) 630 C
C) 835 C
D) 1108 C
E) 1381 C
A) 384 C
B) 630 C
C) 835 C
D) 1108 C
E) 1381 C

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
23
At constant T and P, in which situation will the reaction be product-favored at high temperature but not at low temperature?
A) DH > 0 and DS < 0
B) DH > 0 and DS > 0
C) DH < 0 and DS < 0
D) DH < 0 and DS > 0
E) DH = 0 and DS < 0
A) DH > 0 and DS < 0
B) DH > 0 and DS > 0
C) DH < 0 and DS < 0
D) DH < 0 and DS > 0
E) DH = 0 and DS < 0

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
24
Calculate the value of DS for the reaction shown: 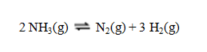 At 25 C the values of entropy in J K-1 mol-1 are ammonia, 192.77; nitrogen, 191.61; and hydrogen, 130.68.
At 25 C the values of entropy in J K-1 mol-1 are ammonia, 192.77; nitrogen, 191.61; and hydrogen, 130.68.
A) +969.19 J/K
B) +393.20 J/K
C) +390.88 J/K
D) +259.03 J/K
E) +198.11 J/K
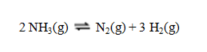 At 25 C the values of entropy in J K-1 mol-1 are ammonia, 192.77; nitrogen, 191.61; and hydrogen, 130.68.
At 25 C the values of entropy in J K-1 mol-1 are ammonia, 192.77; nitrogen, 191.61; and hydrogen, 130.68.A) +969.19 J/K
B) +393.20 J/K
C) +390.88 J/K
D) +259.03 J/K
E) +198.11 J/K

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
25
At constant T and P, in which of the following situations will the reaction never be product-favored?
A) DH > 0 and DS < 0
B) DH > 0 and DS > 0
C) DH < 0 and DS < 0
D) DH < 0 and DS > 0
E) none of these
A) DH > 0 and DS < 0
B) DH > 0 and DS > 0
C) DH < 0 and DS < 0
D) DH < 0 and DS > 0
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
26
Use the data at 25 C given below to calculate the value of DG rxn for the reaction shown when it takes place at 120 C. 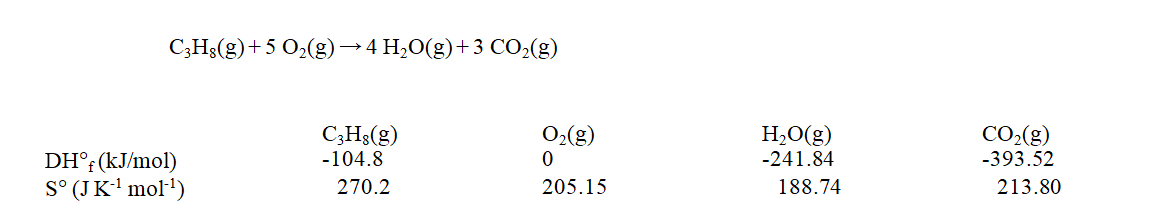
A) -2004 kJ
B) -2046 kJ
C) -2055 kJ
D) -2073 kJ
E) -2083 kJ
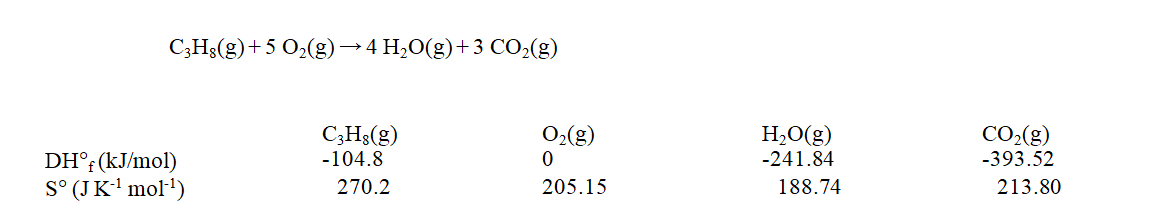
A) -2004 kJ
B) -2046 kJ
C) -2055 kJ
D) -2073 kJ
E) -2083 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
27
According to the Second Law of Thermodynamics,
A) any process during which the entropy of the system increases will be product-favored
B) any process during which the entropy of the universe increases will be product-favored
C) any process which is endothermic will be product-favored
D) the total amount of matter in the universe remains constant
E) heat will always be transferred from a cooler object to a warmer object
A) any process during which the entropy of the system increases will be product-favored
B) any process during which the entropy of the universe increases will be product-favored
C) any process which is endothermic will be product-favored
D) the total amount of matter in the universe remains constant
E) heat will always be transferred from a cooler object to a warmer object

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
28
At constant T and P, in which of the following situations will the reaction always be product-favored?
A) DH > 0 and DS < 0
B) DH > 0 and DS > 0
C) DH < 0 and DS < 0
D) DH < 0 and DS > 0
E) there are no examples of reactions that are always product-favored
A) DH > 0 and DS < 0
B) DH > 0 and DS > 0
C) DH < 0 and DS < 0
D) DH < 0 and DS > 0
E) there are no examples of reactions that are always product-favored

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
29
Which statement is not correct?
A) the aqueous hydrogen ion (H+) is the only species for which DH f, S , and DG f each have a value of zero at 25 C
B) DH f = 0 for each of the elements in its standard state at 25 C
C) DG f = 0 for each of the elements in its standard state at 25 C
D) S ¹ 0 for each of the elements in its standard state at 25 C
E) DH f, S , and DG f each have a value of zero for each of the elements in its standard state at 25 C
A) the aqueous hydrogen ion (H+) is the only species for which DH f, S , and DG f each have a value of zero at 25 C
B) DH f = 0 for each of the elements in its standard state at 25 C
C) DG f = 0 for each of the elements in its standard state at 25 C
D) S ¹ 0 for each of the elements in its standard state at 25 C
E) DH f, S , and DG f each have a value of zero for each of the elements in its standard state at 25 C

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
30
A reaction is exothermic and has a negative value of DS . The value of DG for this reaction is therefore:
A) negative at all temperatures.
B) positive at all temperatures.
C) positive above 0 C and negative below 0 C.
D) positive above a certain temperature and negative below it.
E) negative above a certain temperature and positive below it.
A) negative at all temperatures.
B) positive at all temperatures.
C) positive above 0 C and negative below 0 C.
D) positive above a certain temperature and negative below it.
E) negative above a certain temperature and positive below it.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
31
Use the data given to calculate the value of DG rxn for the reaction at 25 C 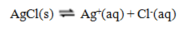

A) -75.2 kJ
B) -55.7 kJ
C) +32.5 kJ
D) +55.7 kJ
E) +75.2 kJ
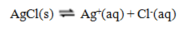

A) -75.2 kJ
B) -55.7 kJ
C) +32.5 kJ
D) +55.7 kJ
E) +75.2 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
32
For a particular reaction, the value of DH = +98.8 kJ and DS = +141.5 J/K. This reaction is
A) product-favored, because DS universe is positive
B) reactant-favored, because DS universe is positive
C) reactant-favored, because DS universe is negative
D) product-favored, because DS universe is negative
E) cannot determine without further information
A) product-favored, because DS universe is positive
B) reactant-favored, because DS universe is positive
C) reactant-favored, because DS universe is negative
D) product-favored, because DS universe is negative
E) cannot determine without further information

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
33
At constant T and P, in which of the following situations will the reaction be product-favored at low temperature but not at high temperature?
A) DH > 0 and DS < 0
B) DH > 0 and DS > 0
C) DH < 0 and DS < 0
D) DH < 0 and DS > 0
E) all product-favored reactions proceed forward at all temperatures
A) DH > 0 and DS < 0
B) DH > 0 and DS > 0
C) DH < 0 and DS < 0
D) DH < 0 and DS > 0
E) all product-favored reactions proceed forward at all temperatures

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
34
Use the data given to calculate the value of DG rxn at 25 C for the reaction given below. DG f for CO(g) is -137.16 kJ/mol. 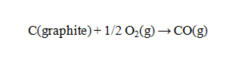
A) -274.32 kJ
B) -137.16 kJ
C) -68.58 kJ
D) +137.16 kJ
E) +274.32 kJ
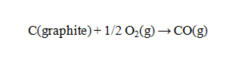
A) -274.32 kJ
B) -137.16 kJ
C) -68.58 kJ
D) +137.16 kJ
E) +274.32 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the value of DS for the reaction shown: 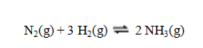 At 25 C the values of entropy in J K-1 mol-1 are nitrogen, 191.61: hydrogen, 130.68; and ammonia, 192.77.
At 25 C the values of entropy in J K-1 mol-1 are nitrogen, 191.61: hydrogen, 130.68; and ammonia, 192.77.
A) -198.11 J/K
B) -259.03 J/K
C) -390.88 J/K
D) -393.20 J/K
E) -969.19 J/K
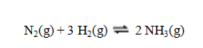 At 25 C the values of entropy in J K-1 mol-1 are nitrogen, 191.61: hydrogen, 130.68; and ammonia, 192.77.
At 25 C the values of entropy in J K-1 mol-1 are nitrogen, 191.61: hydrogen, 130.68; and ammonia, 192.77.A) -198.11 J/K
B) -259.03 J/K
C) -390.88 J/K
D) -393.20 J/K
E) -969.19 J/K

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
36
A slight change in temperature can change the directionality of a reaction when
A) T = DH rxn / DS rxn
B) T = DS rxn / DH rxn
C) DG rxn > 0
D) DG rxn = 0
E) DG rxn is a much larger positive value than DH rxn
A) T = DH rxn / DS rxn
B) T = DS rxn / DH rxn
C) DG rxn > 0
D) DG rxn = 0
E) DG rxn is a much larger positive value than DH rxn

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
37
If a reaction is product-favored at any temperature, then DH is ____ and DS is ____.
A) positive; positive
B) positive; negative
C) zero; positive
D) negative; positive
E) negative; negative
A) positive; positive
B) positive; negative
C) zero; positive
D) negative; positive
E) negative; negative

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
38
For a specific chemical reaction, the value of DG calculated from tabulated values of the standard Gibbs free energy of formation, DG f, of the reactants and products is valid at
A) all temperatures
B) standard temperature and pressure (STP)
C) 0 K
D) 273 K
E) the temperature specified for the tabulated values
A) all temperatures
B) standard temperature and pressure (STP)
C) 0 K
D) 273 K
E) the temperature specified for the tabulated values

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
39
A specific chemical reaction must be product-favored if
A) DG rxn > 0
B) DG rxn = 0
C) DG rxn < 0
D) DH rxn > 0 and DS rxn < 0
E) DH rxn < 0 and DS rxn < 0
A) DG rxn > 0
B) DG rxn = 0
C) DG rxn < 0
D) DH rxn > 0 and DS rxn < 0
E) DH rxn < 0 and DS rxn < 0

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
40
Use the data given to calculate the value of DG rxn for the reaction at 25 C. 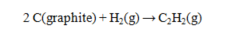

A) -291.4 kJ
B) -244.3 kJ
C) -226.8 kJ
D) -207.6 kJ
E) -64.6 kJ
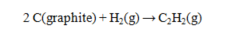

A) -291.4 kJ
B) -244.3 kJ
C) -226.8 kJ
D) -207.6 kJ
E) -64.6 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
41
For the reaction shown, DG = -32.8 kJ at 25 C.
N2(g) + 3 H2(g) ? 2 NH3(g)
a. Calculate the equilibrium constant for this reaction at 25°C.
b. Is a mixture of the three gases where
PN2 = 3.5 bar,
PH2 = 1.2 bar, and
PNH3 = 0.22 bar at equilibrium? Justify your answer.
c. What is the value of DG under the conditions of Part b?
d. Compare the answer from Part c with DG°. Explain the difference observed.
N2(g) + 3 H2(g) ? 2 NH3(g)
a. Calculate the equilibrium constant for this reaction at 25°C.
b. Is a mixture of the three gases where
PN2 = 3.5 bar,
PH2 = 1.2 bar, and
PNH3 = 0.22 bar at equilibrium? Justify your answer.
c. What is the value of DG under the conditions of Part b?
d. Compare the answer from Part c with DG°. Explain the difference observed.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
42
It is possible for a substance, with respect to a given reaction, to
A) be kinetically and thermodynamically stable.
B) be kinetically stable but not thermodynamically stable.
C) be thermodynamically stable but not kinetically stable.
D) fall in either category a or category b.
E) fall in either category a or category c.
A) be kinetically and thermodynamically stable.
B) be kinetically stable but not thermodynamically stable.
C) be thermodynamically stable but not kinetically stable.
D) fall in either category a or category b.
E) fall in either category a or category c.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
43
Which statement correctly describes the meaning of the value of DG rxn for a reactant-favored reaction?
A) It is the amount of energy that is required to overcome the disorder of the system.
B) It is the amount of additional energy that must be supplied in order to initiate reaction.
C) It is the minimum amount of heat released from the system when reaction occurs.
D) It is the maximum amount of useful work obtainable from the reaction when it occurs.
E) It is the minimum amount of work which must be done on the system to make reaction occur.
A) It is the amount of energy that is required to overcome the disorder of the system.
B) It is the amount of additional energy that must be supplied in order to initiate reaction.
C) It is the minimum amount of heat released from the system when reaction occurs.
D) It is the maximum amount of useful work obtainable from the reaction when it occurs.
E) It is the minimum amount of work which must be done on the system to make reaction occur.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
44
If a chemical reaction is at equilibrium, it must be true that
A) DG = 1.
B) DG > 1.
C) DG < 1.
D) DG = 1.
E) DG = 0.
A) DG = 1.
B) DG > 1.
C) DG < 1.
D) DG = 1.
E) DG = 0.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
45
Use the data given to calculate the value of K for the reaction at 25 C. 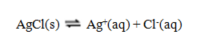

A) 6.61 × 10-14
B) 3.41 × 10-12
C) 4.75 × 10-12
D) 1.76 × 10-10
E) 5.69 × 109
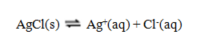

A) 6.61 × 10-14
B) 3.41 × 10-12
C) 4.75 × 10-12
D) 1.76 × 10-10
E) 5.69 × 109

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
46
Use the data given to calculate the value of K for the reaction at 5 C. 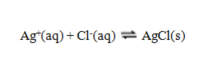

A) 1.9 × 1012
B) 4.5 × 1010
C) 5.7 × 109
D) 1.3 × 106
E) 1.0
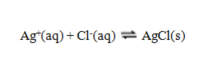

A) 1.9 × 1012
B) 4.5 × 1010
C) 5.7 × 109
D) 1.3 × 106
E) 1.0

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
47
Which set of conditions describes a reaction that is least likely to proceed?
A) endothermic, decreasing entropy, high activation energy
B) exothermic, decreasing entropy, high activation energy
C) exothermic, increasing entropy, high activation energy
D) exothermic, increasing entropy, low activation energy
E) endothermic, decreasing entropy, low activation energy
A) endothermic, decreasing entropy, high activation energy
B) exothermic, decreasing entropy, high activation energy
C) exothermic, increasing entropy, high activation energy
D) exothermic, increasing entropy, low activation energy
E) endothermic, decreasing entropy, low activation energy

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
48
Recovering aluminum directly from its ore, which is primarily aluminum oxide, involves the following reaction, for which thermodynamic data is tabulated below:
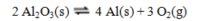

a. Calculate DH°rxn and DS°rxn.
b. Explain how you could have predicted the signs of DH°rxn and DS°rxn without any calculations.
c. Without performing any further calculations, predict whether this reaction will be product-favored only above a certain temperature, or only below a certain temperature. Explain your answer.
d. Calculate the temperature alluded to in Part c.
e. Calculate DG° at this temperature.
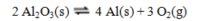

a. Calculate DH°rxn and DS°rxn.
b. Explain how you could have predicted the signs of DH°rxn and DS°rxn without any calculations.
c. Without performing any further calculations, predict whether this reaction will be product-favored only above a certain temperature, or only below a certain temperature. Explain your answer.
d. Calculate the temperature alluded to in Part c.
e. Calculate DG° at this temperature.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
49
Since no chemical process is 100% efficient, some Gibbs free energy is always "wasted" and converted to ____ instead of doing useful work.
A) chemical energy
B) electricity
C) heat
D) light
E) mechanical energy
A) chemical energy
B) electricity
C) heat
D) light
E) mechanical energy

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
50
Which set of conditions describes a reaction that is most likely to proceed?
A) endothermic, decreasing entropy, high activation energy
B) exothermic, decreasing entropy, high activation energy
C) exothermic, increasing entropy, high activation energy
D) exothermic, increasing entropy, low activation energy
E) endothermic, decreasing entropy, low activation energy
A) endothermic, decreasing entropy, high activation energy
B) exothermic, decreasing entropy, high activation energy
C) exothermic, increasing entropy, high activation energy
D) exothermic, increasing entropy, low activation energy
E) endothermic, decreasing entropy, low activation energy

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
51
What is the value of the equilibrium constant at 25 C for a reaction, if the value of DG rxn is -47.8 kJ at 25 C?
A) 1.70
B) 6.88
C) 2.30 × 102
D) 2.74 × 105
E) 2.37 × 108
A) 1.70
B) 6.88
C) 2.30 × 102
D) 2.74 × 105
E) 2.37 × 108

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
52
The value of DG rxn at 45 C is -47.8 kJ. What is the value of the equilibrium constant for this reaction?
A) 1.14
B) 1.02
C) 1.05 × 1024
D) 7.11 × 107
E) 1.40 × 109
A) 1.14
B) 1.02
C) 1.05 × 1024
D) 7.11 × 107
E) 1.40 × 109

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
53
A reaction is product-favored when
A) K < 1 and DG > 0
B) K > 1 and DG < 0
C) K = 1 and DG = 0
D) K = 0 and DG = 0
E) K < 1 and DG = 0
A) K < 1 and DG > 0
B) K > 1 and DG < 0
C) K = 1 and DG = 0
D) K = 0 and DG = 0
E) K < 1 and DG = 0

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
54
If two reactions are coupled, then
A) A product of one reaction is a reactant of the other, and the overall process is reactant-favored.
B) A product of one reaction is a reactant of the other, and the overall process is product-favored.
C) The heat released from an exothermic reaction is used in an endothermic reaction.
D) A product-favored reaction supplies the activation energy for another reaction.
E) The Gibbs free energy released from a product-favored reaction is released to the surroundings, causing an increase in entropy for a second reaction.
A) A product of one reaction is a reactant of the other, and the overall process is reactant-favored.
B) A product of one reaction is a reactant of the other, and the overall process is product-favored.
C) The heat released from an exothermic reaction is used in an endothermic reaction.
D) A product-favored reaction supplies the activation energy for another reaction.
E) The Gibbs free energy released from a product-favored reaction is released to the surroundings, causing an increase in entropy for a second reaction.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
The value of the equilibrium constant for a reaction is 2.65 × 10-6 at 45 C. Calculate the value of DG rxn at this temperature.
A) +47.4 kJ
B) +34.0 kJ
C) +14.8 kJ
D) +335 kJ
E) -14.8 kJ
A) +47.4 kJ
B) +34.0 kJ
C) +14.8 kJ
D) +335 kJ
E) -14.8 kJ

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
56
The enthalpy change for a reaction can be calculated using the equation
DH°rxn It would be possible to use DS f values to calculate DS rxn in a similar manner; however, this is not done in practice.
a. Describe the procedure which is used instead.
b. Explain why this mathematical process is possible for entropy calculations but not for enthalpy calculations.
DH°rxn It would be possible to use DS f values to calculate DS rxn in a similar manner; however, this is not done in practice.
a. Describe the procedure which is used instead.
b. Explain why this mathematical process is possible for entropy calculations but not for enthalpy calculations.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck


