Exam 17: Thermodynamics: Directionality of Chemical Reactions
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
Use the data given to calculate the value of DG rxn for the reaction at 25 C. 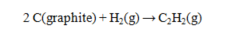

Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
B
According to the Second Law of Thermodynamics,
Free
(Multiple Choice)
4.9/5  (44)
(44)
Correct Answer:
B
For the reaction shown, DG = -32.8 kJ at 25 C.
N2(g) + 3 H2(g) ? 2 NH3(g)
a. Calculate the equilibrium constant for this reaction at 25°C.
b. Is a mixture of the three gases where
PN2 = 3.5 bar,
PH2 = 1.2 bar, and
PNH3 = 0.22 bar at equilibrium? Justify your answer.
c. What is the value of DG under the conditions of Part b?
d. Compare the answer from Part c with DG°. Explain the difference observed.
(Essay)
4.7/5  (28)
(28)
Recovering aluminum directly from its ore, which is primarily aluminum oxide, involves the following reaction, for which thermodynamic data is tabulated below:
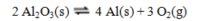
 a. Calculate DH°rxn and DS°rxn.
b. Explain how you could have predicted the signs of DH°rxn and DS°rxn without any calculations.
c. Without performing any further calculations, predict whether this reaction will be product-favored only above a certain temperature, or only below a certain temperature. Explain your answer.
d. Calculate the temperature alluded to in Part c.
e. Calculate DG° at this temperature.
a. Calculate DH°rxn and DS°rxn.
b. Explain how you could have predicted the signs of DH°rxn and DS°rxn without any calculations.
c. Without performing any further calculations, predict whether this reaction will be product-favored only above a certain temperature, or only below a certain temperature. Explain your answer.
d. Calculate the temperature alluded to in Part c.
e. Calculate DG° at this temperature.
(Essay)
4.8/5  (27)
(27)
At constant T and P, in which of the following situations will the reaction be product-favored at low temperature but not at high temperature?
(Multiple Choice)
4.8/5  (44)
(44)
Calculate the value of DS for the reaction shown: 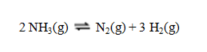 At 25 C the values of entropy in J K-1 mol-1 are ammonia, 192.77; nitrogen, 191.61; and hydrogen, 130.68.
At 25 C the values of entropy in J K-1 mol-1 are ammonia, 192.77; nitrogen, 191.61; and hydrogen, 130.68.
(Multiple Choice)
4.8/5  (39)
(39)
If a chemical reaction is at equilibrium, it must be true that
(Multiple Choice)
4.8/5  (39)
(39)
Which set of conditions describes a reaction that is most likely to proceed?
(Multiple Choice)
4.9/5  (37)
(37)
Which statement correctly describes the meaning of the value of DG rxn for a reactant-favored reaction?
(Multiple Choice)
4.8/5  (33)
(33)
The enthalpy change for a reaction can be calculated using the equation
DH°rxn It would be possible to use DS f values to calculate DS rxn in a similar manner; however, this is not done in practice.
a. Describe the procedure which is used instead.
b. Explain why this mathematical process is possible for entropy calculations but not for enthalpy calculations.
(Essay)
4.8/5  (32)
(32)
Use the data at 25 C given below to calculate the value of DG rxn for the reaction shown when it takes place at 120 C. 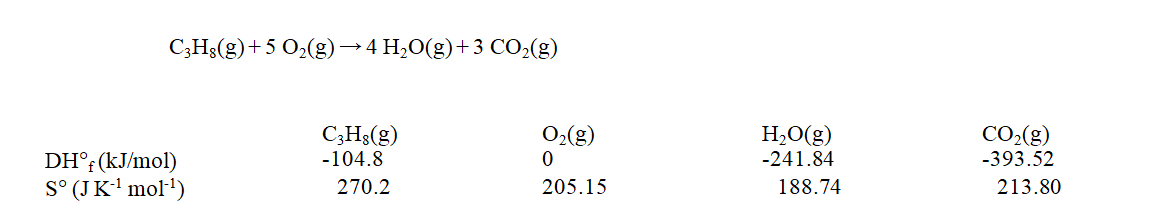
(Multiple Choice)
4.8/5  (40)
(40)
A certain reaction has DH rxn = +177.8 kJ, and DS rxn = +160.5 J/K. Above what temperature does it become product-favored ?
(Multiple Choice)
4.9/5  (46)
(46)
At constant T and P, in which of the following situations will the reaction never be product-favored?
(Multiple Choice)
4.8/5  (38)
(38)
Nature favors exothermic reactions because after such a reaction
(Multiple Choice)
4.8/5  (28)
(28)
What is the value of the equilibrium constant at 25 C for a reaction, if the value of DG rxn is -47.8 kJ at 25 C?
(Multiple Choice)
4.8/5  (34)
(34)
Which process involves a decrease of entropy for the system?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 1 - 20 of 56
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)