Deck 13: Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/57
Play
Full screen (f)
Deck 13: Chemical Equilibrium
1
The equilibrium constant expression for the reaction shown below is 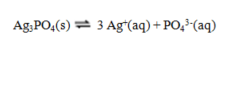
A)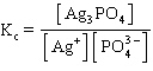
B)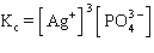
C)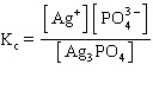
D)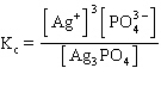
E)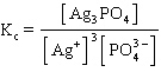
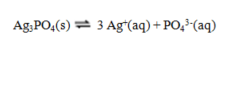
A)
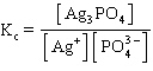
B)
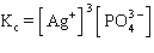
C)
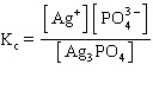
D)
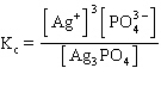
E)
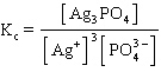
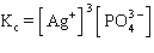
2
Consider the equilibrium reaction 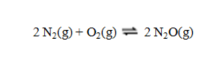 In a particular experiment, the equilibrium concentration of N2 is 0.048 M, of O2, 0.093 M, and of N2O, 6.55 × 10-21 M. What is the value of the equilibrium constant Kc?
In a particular experiment, the equilibrium concentration of N2 is 0.048 M, of O2, 0.093 M, and of N2O, 6.55 × 10-21 M. What is the value of the equilibrium constant Kc?
A) 1.5 × 10-18
B) 2.0 × 10-37
C) 2.2 × 10-36
D) 3.1 × 10-17
E) 5.0 × 1036
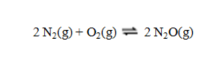 In a particular experiment, the equilibrium concentration of N2 is 0.048 M, of O2, 0.093 M, and of N2O, 6.55 × 10-21 M. What is the value of the equilibrium constant Kc?
In a particular experiment, the equilibrium concentration of N2 is 0.048 M, of O2, 0.093 M, and of N2O, 6.55 × 10-21 M. What is the value of the equilibrium constant Kc?A) 1.5 × 10-18
B) 2.0 × 10-37
C) 2.2 × 10-36
D) 3.1 × 10-17
E) 5.0 × 1036
2.0 × 10-37
3
Terms relating to certain types of substances are conventionally omitted from equilibrium constant expressions. These substances are
A) pure liquids and gases.
B) pure solids and liquids.
C) pure solids only.
D) pure liquids only.
E) pure solids, liquids and gases.
A) pure liquids and gases.
B) pure solids and liquids.
C) pure solids only.
D) pure liquids only.
E) pure solids, liquids and gases.
pure solids and liquids.
4
Consider the gas-phase equilibrium A  B. In a series of experiments, different initial amounts of A and B are mixed together, and the mixture in each case allowed to come to equilibrium. Which one of these experiments would yield values for the amounts of A and B present at equilibrium different from all the other experiments?
B. In a series of experiments, different initial amounts of A and B are mixed together, and the mixture in each case allowed to come to equilibrium. Which one of these experiments would yield values for the amounts of A and B present at equilibrium different from all the other experiments?
A) 3.0 moles A, 4.5 moles B
B) 4.5 moles A, 3.0 moles B
C) 1.5 moles A, 4.5 moles B
D) 7.5 moles A, no B
E) 0.5 moles A, 7.0 moles B
 B. In a series of experiments, different initial amounts of A and B are mixed together, and the mixture in each case allowed to come to equilibrium. Which one of these experiments would yield values for the amounts of A and B present at equilibrium different from all the other experiments?
B. In a series of experiments, different initial amounts of A and B are mixed together, and the mixture in each case allowed to come to equilibrium. Which one of these experiments would yield values for the amounts of A and B present at equilibrium different from all the other experiments?A) 3.0 moles A, 4.5 moles B
B) 4.5 moles A, 3.0 moles B
C) 1.5 moles A, 4.5 moles B
D) 7.5 moles A, no B
E) 0.5 moles A, 7.0 moles B

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
5
The equilibrium constant expression for the reverse of the reaction given below is 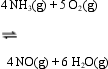
A)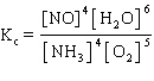
B)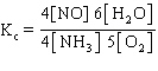
C)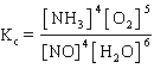
D)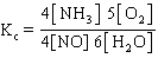
E)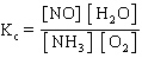
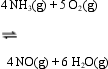
A)
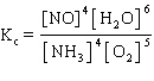
B)
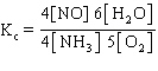
C)
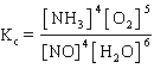
D)
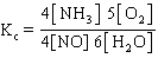
E)
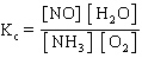

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
6
A particular chemical reaction is
A) reactant-favored if the equilibrium constant is very large
B) product-favored if the equilibrium constant is very small
C) either product-favored or reactant-favored depending on the temperature of the reaction
D) either product-favored or reactant-favored depending on the pressure of the reaction
E) all of these
A) reactant-favored if the equilibrium constant is very large
B) product-favored if the equilibrium constant is very small
C) either product-favored or reactant-favored depending on the temperature of the reaction
D) either product-favored or reactant-favored depending on the pressure of the reaction
E) all of these

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
7
The equilibrium constant for a reaction is related to the rate constants for the reaction as follows:
A) K = kreverse / kforward
B) K = kforward / kreverse
C) K = kforward × kreverse
D) K = kforward
E) K = kreverse
A) K = kreverse / kforward
B) K = kforward / kreverse
C) K = kforward × kreverse
D) K = kforward
E) K = kreverse

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
8
Consider the gas-phase equilibrium A  B. Certain amounts of A and B are mixed in a vessel. As they come to equilibrium
B. Certain amounts of A and B are mixed in a vessel. As they come to equilibrium
A) the forward reaction rate declines and the reverse reaction rate rises.
B) the reverse reaction rate declines and the forward reaction rate rises.
C) both forward and reverse reaction rates decline.
D) both forward and reverse reaction rates rise.
E) either a or b, but we cannot say which without more information.
 B. Certain amounts of A and B are mixed in a vessel. As they come to equilibrium
B. Certain amounts of A and B are mixed in a vessel. As they come to equilibriumA) the forward reaction rate declines and the reverse reaction rate rises.
B) the reverse reaction rate declines and the forward reaction rate rises.
C) both forward and reverse reaction rates decline.
D) both forward and reverse reaction rates rise.
E) either a or b, but we cannot say which without more information.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
9
The equilibrium for a particular chemical reaction is dependent on the
A) homogeneous catalyst concentration, if catalyst is present
B) direction of approach
C) temperature of the reaction
D) atmospheric pressure
E) initial concentrations of the reactants
A) homogeneous catalyst concentration, if catalyst is present
B) direction of approach
C) temperature of the reaction
D) atmospheric pressure
E) initial concentrations of the reactants

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
10
The equilibrium constant for the reaction 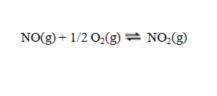 has a value of Kc = 1.23 at a certain temperature. What is the value of Kc for the reaction:
has a value of Kc = 1.23 at a certain temperature. What is the value of Kc for the reaction:
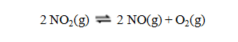
A) 2.46
B) 1.51
C) 0.66
D) 0.41
E) -1.51
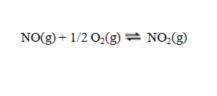 has a value of Kc = 1.23 at a certain temperature. What is the value of Kc for the reaction:
has a value of Kc = 1.23 at a certain temperature. What is the value of Kc for the reaction: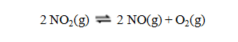
A) 2.46
B) 1.51
C) 0.66
D) 0.41
E) -1.51

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
11
In a balanced chemical equation, if there is no net change in the total number of moles of gas from the reactant side to the product side, then
A) Kp = 1.00
B) Kp = Kc
C) Kc = 1.00
D) at equilibrium, the reaction has gone to 50% completion
E) all of these
A) Kp = 1.00
B) Kp = Kc
C) Kc = 1.00
D) at equilibrium, the reaction has gone to 50% completion
E) all of these

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
12
The concentration equilibrium constant, Kc, and the pressure equilibrium constant, Kp, are related by the expression
A) Kc = Kp(RT)Dn
B) Kc = Kp(Dn)RT
C) Kp = Kc(RT)Dn
D) Kp = Kc(R)TDn
E) Kp = Kc(Dn)RT
A) Kc = Kp(RT)Dn
B) Kc = Kp(Dn)RT
C) Kp = Kc(RT)Dn
D) Kp = Kc(R)TDn
E) Kp = Kc(Dn)RT

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
13
A weak acid is 5% ionized at equilibrium. Therefore we can say that the ionization reaction is ____-favored, because ____.
A) product; the amount of products << the amount of reactants
B) reactant; the amount of products << the amount of reactants
C) reactant; the amount of products >> the amount of reactants
D) product; the amount of products >> the amount of reactants
E) neither; not enough information is available to reach a conclusion
A) product; the amount of products << the amount of reactants
B) reactant; the amount of products << the amount of reactants
C) reactant; the amount of products >> the amount of reactants
D) product; the amount of products >> the amount of reactants
E) neither; not enough information is available to reach a conclusion

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
14
Consider the equilibrium reaction 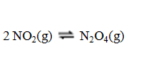 A sample of pure NO2(g) of concentration 0.140 M is allowed to come to equilibrium. It is then found that 57.0 % of the NO2(g) has reacted to form N2O4(g). What is the value of Kc?
A sample of pure NO2(g) of concentration 0.140 M is allowed to come to equilibrium. It is then found that 57.0 % of the NO2(g) has reacted to form N2O4(g). What is the value of Kc?
A) 0.211
B) 0.377
C) 0.754
D) 4.73
E) 11.0
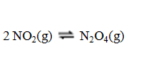 A sample of pure NO2(g) of concentration 0.140 M is allowed to come to equilibrium. It is then found that 57.0 % of the NO2(g) has reacted to form N2O4(g). What is the value of Kc?
A sample of pure NO2(g) of concentration 0.140 M is allowed to come to equilibrium. It is then found that 57.0 % of the NO2(g) has reacted to form N2O4(g). What is the value of Kc?A) 0.211
B) 0.377
C) 0.754
D) 4.73
E) 11.0

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
15
If the equilibrium constants for the two reactions 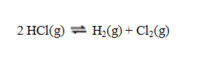 and
and
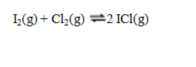
are denoted K1 and K2 respectively, then the equilibrium constant for the reaction
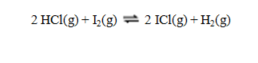 equals
equals
A) (K1/K2)2.
B) (K1K2)2.
C) K1K2.
D) K1 + K2.
E) K1K2/2.
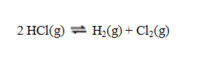 and
and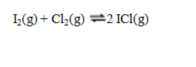
are denoted K1 and K2 respectively, then the equilibrium constant for the reaction
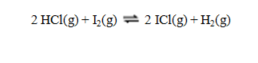 equals
equalsA) (K1/K2)2.
B) (K1K2)2.
C) K1K2.
D) K1 + K2.
E) K1K2/2.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
16
A chemical equilibrium may involve
A) reactants and products exhibiting different states of matter
B) molecular rearrangements
C) a chemical change of one compound into another
D) reaction of elements to produce one or more compounds
E) any of these
A) reactants and products exhibiting different states of matter
B) molecular rearrangements
C) a chemical change of one compound into another
D) reaction of elements to produce one or more compounds
E) any of these

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
17
The equilibrium constant for a particular chemical reaction is
A) dependent on the total pressure of the reaction
B) dependent on the temperature of the reaction
C) a numerical value between -¥ and +¥
D) unreliably determined if variable amounts of either solid or liquid reactants or products are present
E) both b and c
A) dependent on the total pressure of the reaction
B) dependent on the temperature of the reaction
C) a numerical value between -¥ and +¥
D) unreliably determined if variable amounts of either solid or liquid reactants or products are present
E) both b and c

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
18
If a catalyst is added to a chemical reaction, the equilibrium yield of a product will be ____, and the time taken to come to equilibrium will be ____ than before.
A) higher; less
B) lower; the same
C) higher; the same
D) the same; less
E) lower; less
A) higher; less
B) lower; the same
C) higher; the same
D) the same; less
E) lower; less

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is not true for equilibrium constants?
A) when related reactions are added together, their equilibrium constants are added together
B) the equilibrium constants of the forward and reverse reactions are negative reciprocals of one another
C) when the coefficients in a balanced chemical reaction are multiplied by a factor, the equilibrium constant is multiplied by the same factor
D) the equilibrium constants of the forward and reverse reactions are equal to the respective rate constants of these reactions
E) all of these
A) when related reactions are added together, their equilibrium constants are added together
B) the equilibrium constants of the forward and reverse reactions are negative reciprocals of one another
C) when the coefficients in a balanced chemical reaction are multiplied by a factor, the equilibrium constant is multiplied by the same factor
D) the equilibrium constants of the forward and reverse reactions are equal to the respective rate constants of these reactions
E) all of these

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
20
A chemical reaction reaches equilibrium when
A) both the forward and reverse reactions stop
B) the forward and reverse reactions both occur at the same rate
C) all of the limiting reactant has been used up
D) all of the limiting reactant has been used up and all of the limiting product has been created
E) the actual yield of the reaction equals the theoretical yield
A) both the forward and reverse reactions stop
B) the forward and reverse reactions both occur at the same rate
C) all of the limiting reactant has been used up
D) all of the limiting reactant has been used up and all of the limiting product has been created
E) the actual yield of the reaction equals the theoretical yield

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the equilibrium system 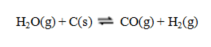 If C is removed, the equilibrium will ____, and if CO is added, the equilibrium will ____.
If C is removed, the equilibrium will ____, and if CO is added, the equilibrium will ____.
A) shift to the left; shift to the left
B) shift to the right; shift to the right
C) shift to the right; shift to the left
D) be unchanged; shift to the left
E) be unchanged; shift to the right
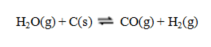 If C is removed, the equilibrium will ____, and if CO is added, the equilibrium will ____.
If C is removed, the equilibrium will ____, and if CO is added, the equilibrium will ____.A) shift to the left; shift to the left
B) shift to the right; shift to the right
C) shift to the right; shift to the left
D) be unchanged; shift to the left
E) be unchanged; shift to the right

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
22
To decide whether a reaction mixture is at equilibrium, a student determines the value of Q, the reaction quotient, and finds that it is less than K. Therefore, the mixture is
A) at equilibrium, since there is as much reaction as required.
B) not at equilibrium, and will react to the right, to increase the amounts of products.
C) not at equilibrium, and will react to the left, to increase the amounts of reactants.
D) not at equilibrium, and will react to the right, to increase the amounts of reactants.
E) not at equilibrium, and will react to the left, to increase the amounts of products.
A) at equilibrium, since there is as much reaction as required.
B) not at equilibrium, and will react to the right, to increase the amounts of products.
C) not at equilibrium, and will react to the left, to increase the amounts of reactants.
D) not at equilibrium, and will react to the right, to increase the amounts of reactants.
E) not at equilibrium, and will react to the left, to increase the amounts of products.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
23
An endothermic reaction which results in an increase in moles of gas will be most product-favored under conditions of ____ pressure and ____ temperature.
A) high; high
B) high; moderate
C) high; low
D) low; high
E) low; low
A) high; high
B) high; moderate
C) high; low
D) low; high
E) low; low

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
24
Consider the reaction 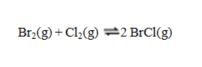 If the partial pressures in an equilibrium mixture of Br2, Cl2, and BrCl are 1.12 atm, 1.26 atm, and 3.14 atm, respectively, the value of Kp for this reaction at this temperature is
If the partial pressures in an equilibrium mixture of Br2, Cl2, and BrCl are 1.12 atm, 1.26 atm, and 3.14 atm, respectively, the value of Kp for this reaction at this temperature is
A) 6.99
B) 4.70
C) 1.91
D) 0.532
E) 0.142
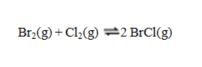 If the partial pressures in an equilibrium mixture of Br2, Cl2, and BrCl are 1.12 atm, 1.26 atm, and 3.14 atm, respectively, the value of Kp for this reaction at this temperature is
If the partial pressures in an equilibrium mixture of Br2, Cl2, and BrCl are 1.12 atm, 1.26 atm, and 3.14 atm, respectively, the value of Kp for this reaction at this temperature isA) 6.99
B) 4.70
C) 1.91
D) 0.532
E) 0.142

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
25
Consider the endothermic reaction 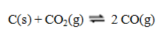 If such a system at equilibrium is heated, equilibrium will ____, because ____.
If such a system at equilibrium is heated, equilibrium will ____, because ____.
A) be unchanged; temperature has no effect on equilibrium
B) shift to the left; increased temperature favors an exothermic reaction
C) shift to the right; increased temperature favors an exothermic reaction
D) shift to the right; increased temperature favors an endothermic reaction
E) shift to the left; increased temperature favors an endothermic reaction
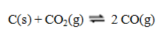 If such a system at equilibrium is heated, equilibrium will ____, because ____.
If such a system at equilibrium is heated, equilibrium will ____, because ____.A) be unchanged; temperature has no effect on equilibrium
B) shift to the left; increased temperature favors an exothermic reaction
C) shift to the right; increased temperature favors an exothermic reaction
D) shift to the right; increased temperature favors an endothermic reaction
E) shift to the left; increased temperature favors an endothermic reaction

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is false?
A) a large equilibrium constant is indicative of a reactant-favored reaction
B) an equilibrium constant close to 1.00 always indicates a highly product-favored reaction
C) if Kp = Kc the reaction has gone to 100% completion at equilibrium
D) if Kp = Kc = 0.50, the reaction has gone to 50% completion at equilibrium
E) all of these
A) a large equilibrium constant is indicative of a reactant-favored reaction
B) an equilibrium constant close to 1.00 always indicates a highly product-favored reaction
C) if Kp = Kc the reaction has gone to 100% completion at equilibrium
D) if Kp = Kc = 0.50, the reaction has gone to 50% completion at equilibrium
E) all of these

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
27
For the reaction ![<strong>For the reaction K<sub>c</sub> = 1.4 × 10<sup>15</sup>. In an equilibrium mixture, [A] = 0.45 M. What is the concentration of B?</strong> A) 2.8 × 10<sup>14</sup> B) 0.90 C) 1.1 × 10<sup>15</sup> D) 6.3 × 10<sup>14</sup> E) 2.5 × 10<sup>7</sup>](https://storage.examlex.com/TB5061/11ea782d_fd80_0c1b_bbd5_bf24949c08e1_TB5061_11.jpg) Kc = 1.4 × 1015. In an equilibrium mixture, [A] = 0.45 M. What is the concentration of B?
Kc = 1.4 × 1015. In an equilibrium mixture, [A] = 0.45 M. What is the concentration of B?
A) 2.8 × 1014
B) 0.90
C) 1.1 × 1015
D) 6.3 × 1014
E) 2.5 × 107
![<strong>For the reaction K<sub>c</sub> = 1.4 × 10<sup>15</sup>. In an equilibrium mixture, [A] = 0.45 M. What is the concentration of B?</strong> A) 2.8 × 10<sup>14</sup> B) 0.90 C) 1.1 × 10<sup>15</sup> D) 6.3 × 10<sup>14</sup> E) 2.5 × 10<sup>7</sup>](https://storage.examlex.com/TB5061/11ea782d_fd80_0c1b_bbd5_bf24949c08e1_TB5061_11.jpg) Kc = 1.4 × 1015. In an equilibrium mixture, [A] = 0.45 M. What is the concentration of B?
Kc = 1.4 × 1015. In an equilibrium mixture, [A] = 0.45 M. What is the concentration of B?A) 2.8 × 1014
B) 0.90
C) 1.1 × 1015
D) 6.3 × 1014
E) 2.5 × 107

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the reaction 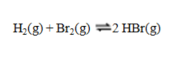 If the partial pressures in an equilibrium mixture of H2, Br2, and HBr are 0.024 atm, 0.031 atm, and 5.07 atm, respectively, the value of Kp for this reaction at this temperature is
If the partial pressures in an equilibrium mixture of H2, Br2, and HBr are 0.024 atm, 0.031 atm, and 5.07 atm, respectively, the value of Kp for this reaction at this temperature is
A) 3.5 × 104
B) 1.4 × 104
C) 6.8 × 103
D) 3.7 × 102
E) 1.9 × 102
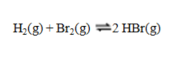 If the partial pressures in an equilibrium mixture of H2, Br2, and HBr are 0.024 atm, 0.031 atm, and 5.07 atm, respectively, the value of Kp for this reaction at this temperature is
If the partial pressures in an equilibrium mixture of H2, Br2, and HBr are 0.024 atm, 0.031 atm, and 5.07 atm, respectively, the value of Kp for this reaction at this temperature isA) 3.5 × 104
B) 1.4 × 104
C) 6.8 × 103
D) 3.7 × 102
E) 1.9 × 102

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
29
For the reaction 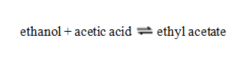 Kc = 0.95. A mixture of the three substances contains 0.45 M ethanol, 0.45 M acetic acid and 1.1 M ethyl acetate. Which statement is true?
Kc = 0.95. A mixture of the three substances contains 0.45 M ethanol, 0.45 M acetic acid and 1.1 M ethyl acetate. Which statement is true?
A) Q < K, so the system will react left to right.
B) Q < K, so the system will react right to left.
C) The mixture is at equilibrium.
D) Q > K, so the system will react left to right.
E) Q > K, so the system will react right to left.
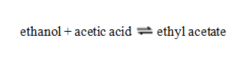 Kc = 0.95. A mixture of the three substances contains 0.45 M ethanol, 0.45 M acetic acid and 1.1 M ethyl acetate. Which statement is true?
Kc = 0.95. A mixture of the three substances contains 0.45 M ethanol, 0.45 M acetic acid and 1.1 M ethyl acetate. Which statement is true?A) Q < K, so the system will react left to right.
B) Q < K, so the system will react right to left.
C) The mixture is at equilibrium.
D) Q > K, so the system will react left to right.
E) Q > K, so the system will react right to left.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
30
If the reaction quotient, Q, is greater than the equilibrium constant, K, then
A) the forward reaction must be favored
B) the reaction will proceed in the forward direction
C) the reaction has gone to 100% completion and is at equilibrium
D) the reaction has not yet started in the forward direction
E) the reaction will proceed in the reverse direction
A) the forward reaction must be favored
B) the reaction will proceed in the forward direction
C) the reaction has gone to 100% completion and is at equilibrium
D) the reaction has not yet started in the forward direction
E) the reaction will proceed in the reverse direction

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
31
For the reaction 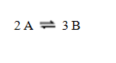 Kc = 1.37. If the concentrations of A and B are equal, what is the value of that concentration?
Kc = 1.37. If the concentrations of A and B are equal, what is the value of that concentration?
A) 0.685 M
B) 0.822 M
C) 1.17 M
D) 1.37 M
E) 1.88 M
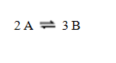 Kc = 1.37. If the concentrations of A and B are equal, what is the value of that concentration?
Kc = 1.37. If the concentrations of A and B are equal, what is the value of that concentration?A) 0.685 M
B) 0.822 M
C) 1.17 M
D) 1.37 M
E) 1.88 M

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
32
Once the reaction quotient, Q, has been determined for a reaction mixture, comparison with the value of the equilibrium constant, K, will determine
A) if the mixture is at equilibrium or not.
B) if the mixture has an excess of either products or reactants compared to equilibrium.
C) if the mixture will react to the left, to the right, or not at all.
D) Both a and b.
E) All of a, b, and c.
A) if the mixture is at equilibrium or not.
B) if the mixture has an excess of either products or reactants compared to equilibrium.
C) if the mixture will react to the left, to the right, or not at all.
D) Both a and b.
E) All of a, b, and c.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
33
For the reaction ![<strong>For the reaction K<sub>c</sub> = 168. A mixture contains some C(s), [CO] = 0.50 M and [CO<sub>2</sub>] = 0.75 M. Therefore the system ____ at equilibrium, because ____.</strong> A) is not; the value of Q is 0.67 B) is not; the value of Q is 1.5 C) is; the value of Q is 0.67 D) is not; the value of Q is 0.33 E) is; the value of Q is 0.33](https://storage.examlex.com/TB5061/11ea782d_fd7f_e509_bbd5_4d147749917b_TB5061_11.jpg) Kc = 168. A mixture contains some C(s), [CO] = 0.50 M and [CO2] = 0.75 M. Therefore the system ____ at equilibrium, because ____.
Kc = 168. A mixture contains some C(s), [CO] = 0.50 M and [CO2] = 0.75 M. Therefore the system ____ at equilibrium, because ____.
A) is not; the value of Q is 0.67
B) is not; the value of Q is 1.5
C) is; the value of Q is 0.67
D) is not; the value of Q is 0.33
E) is; the value of Q is 0.33
![<strong>For the reaction K<sub>c</sub> = 168. A mixture contains some C(s), [CO] = 0.50 M and [CO<sub>2</sub>] = 0.75 M. Therefore the system ____ at equilibrium, because ____.</strong> A) is not; the value of Q is 0.67 B) is not; the value of Q is 1.5 C) is; the value of Q is 0.67 D) is not; the value of Q is 0.33 E) is; the value of Q is 0.33](https://storage.examlex.com/TB5061/11ea782d_fd7f_e509_bbd5_4d147749917b_TB5061_11.jpg) Kc = 168. A mixture contains some C(s), [CO] = 0.50 M and [CO2] = 0.75 M. Therefore the system ____ at equilibrium, because ____.
Kc = 168. A mixture contains some C(s), [CO] = 0.50 M and [CO2] = 0.75 M. Therefore the system ____ at equilibrium, because ____.A) is not; the value of Q is 0.67
B) is not; the value of Q is 1.5
C) is; the value of Q is 0.67
D) is not; the value of Q is 0.33
E) is; the value of Q is 0.33

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the reaction 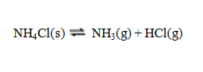 If an equilibrium mixture of these three substances is compressed, equilibrium will ____, because ____.
If an equilibrium mixture of these three substances is compressed, equilibrium will ____, because ____.
A) shift to the right; higher pressure favors fewer moles of gas
B) shift top the right; higher pressure favors more moles of gas
C) shift to the left; higher pressure favors fewer moles of gas
D) shift to the left; higher pressure favors more moles of gas
E) be unchanged; solid NH4Cl does not appear in the equilibrium constant expression.
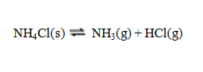 If an equilibrium mixture of these three substances is compressed, equilibrium will ____, because ____.
If an equilibrium mixture of these three substances is compressed, equilibrium will ____, because ____.A) shift to the right; higher pressure favors fewer moles of gas
B) shift top the right; higher pressure favors more moles of gas
C) shift to the left; higher pressure favors fewer moles of gas
D) shift to the left; higher pressure favors more moles of gas
E) be unchanged; solid NH4Cl does not appear in the equilibrium constant expression.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
35
For the reaction ![<strong>For the reaction K<sub>c</sub> = 0.0600 at a certain temperature. In an equilibrium mixture of the three gases, [NH<sub>3</sub>] = 0.242 M and [H<sub>2</sub>] = 1.03 M. What is the concentration of N<sub>2</sub> in this system?</strong> A) 3.9 M B) 3.2 × 10<sup>-3</sup> M C) 0.89 M D) 1.4 × 10<sup>-2</sup> M E) 3.7 M](https://storage.examlex.com/TB5061/11ea782d_fd7f_e50a_bbd5_fb3cb3f4e02b_TB5061_11.jpg) Kc = 0.0600 at a certain temperature. In an equilibrium mixture of the three gases, [NH3] = 0.242 M and [H2] = 1.03 M. What is the concentration of N2 in this system?
Kc = 0.0600 at a certain temperature. In an equilibrium mixture of the three gases, [NH3] = 0.242 M and [H2] = 1.03 M. What is the concentration of N2 in this system?
A) 3.9 M
B) 3.2 × 10-3 M
C) 0.89 M
D) 1.4 × 10-2 M
E) 3.7 M
![<strong>For the reaction K<sub>c</sub> = 0.0600 at a certain temperature. In an equilibrium mixture of the three gases, [NH<sub>3</sub>] = 0.242 M and [H<sub>2</sub>] = 1.03 M. What is the concentration of N<sub>2</sub> in this system?</strong> A) 3.9 M B) 3.2 × 10<sup>-3</sup> M C) 0.89 M D) 1.4 × 10<sup>-2</sup> M E) 3.7 M](https://storage.examlex.com/TB5061/11ea782d_fd7f_e50a_bbd5_fb3cb3f4e02b_TB5061_11.jpg) Kc = 0.0600 at a certain temperature. In an equilibrium mixture of the three gases, [NH3] = 0.242 M and [H2] = 1.03 M. What is the concentration of N2 in this system?
Kc = 0.0600 at a certain temperature. In an equilibrium mixture of the three gases, [NH3] = 0.242 M and [H2] = 1.03 M. What is the concentration of N2 in this system?A) 3.9 M
B) 3.2 × 10-3 M
C) 0.89 M
D) 1.4 × 10-2 M
E) 3.7 M

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the equilibrium system 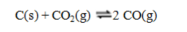 If more C(s) is added, the equilibrium will ____; if CO is removed the equilibrium will ____.
If more C(s) is added, the equilibrium will ____; if CO is removed the equilibrium will ____.
A) shift to the left; shift to the left
B) shift to the right; shift to the right
C) shift to the right; shift to the left
D) be unchanged; shift to the left
E) be unchanged; shift to the right
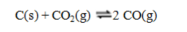 If more C(s) is added, the equilibrium will ____; if CO is removed the equilibrium will ____.
If more C(s) is added, the equilibrium will ____; if CO is removed the equilibrium will ____.A) shift to the left; shift to the left
B) shift to the right; shift to the right
C) shift to the right; shift to the left
D) be unchanged; shift to the left
E) be unchanged; shift to the right

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
37
A particular reaction mixture (Kp = 10) consisting of both gaseous reactants and products is analyzed and is determined to have a value of Q = 15. The pressure of the reaction mixture is then doubled by the addition of argon gas and the reaction is allowed to proceed to equilibrium. At equilibrium
A) Kp > 10
B) Q = 15
C) Q = 10
D) Kp < 10
E) Q = 30
A) Kp > 10
B) Q = 15
C) Q = 10
D) Kp < 10
E) Q = 30

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the reaction ![<strong>Consider the reaction At equilibrium, [CO] = 4.14 × 10<sup>-6</sup> M; [Cl<sub>2</sub>] = 4.14 × 10<sup>-6</sup> M; and [COCl<sub>2</sub>] = 0.0627 M. Calculate the value of the equilibrium constant, K<sub>c</sub>.</strong> A) 2.73 × 10<sup>-10</sup> B) 6.60 × 10<sup>-5</sup> C) 1.32 × 10<sup>-4</sup> D) 1.51 × 10<sup>4</sup> E) 3.66 × 10<sup>9</sup>](https://storage.examlex.com/TB5061/11ec6ef9_ba27_6a04_8fe2_8d74aeeb9d71_TB5061_00.jpg) At equilibrium, [CO] = 4.14 × 10-6 M; [Cl2] = 4.14 × 10-6 M; and [COCl2] = 0.0627 M. Calculate the value of the equilibrium constant, Kc.
At equilibrium, [CO] = 4.14 × 10-6 M; [Cl2] = 4.14 × 10-6 M; and [COCl2] = 0.0627 M. Calculate the value of the equilibrium constant, Kc.
A) 2.73 × 10-10
B) 6.60 × 10-5
C) 1.32 × 10-4
D) 1.51 × 104
E) 3.66 × 109
![<strong>Consider the reaction At equilibrium, [CO] = 4.14 × 10<sup>-6</sup> M; [Cl<sub>2</sub>] = 4.14 × 10<sup>-6</sup> M; and [COCl<sub>2</sub>] = 0.0627 M. Calculate the value of the equilibrium constant, K<sub>c</sub>.</strong> A) 2.73 × 10<sup>-10</sup> B) 6.60 × 10<sup>-5</sup> C) 1.32 × 10<sup>-4</sup> D) 1.51 × 10<sup>4</sup> E) 3.66 × 10<sup>9</sup>](https://storage.examlex.com/TB5061/11ec6ef9_ba27_6a04_8fe2_8d74aeeb9d71_TB5061_00.jpg) At equilibrium, [CO] = 4.14 × 10-6 M; [Cl2] = 4.14 × 10-6 M; and [COCl2] = 0.0627 M. Calculate the value of the equilibrium constant, Kc.
At equilibrium, [CO] = 4.14 × 10-6 M; [Cl2] = 4.14 × 10-6 M; and [COCl2] = 0.0627 M. Calculate the value of the equilibrium constant, Kc.A) 2.73 × 10-10
B) 6.60 × 10-5
C) 1.32 × 10-4
D) 1.51 × 104
E) 3.66 × 109

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
39
Consider the reaction 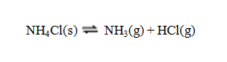 If the pressure is decreased on an equilibrium mixture of these three substances, equilibrium will ____, because ____.
If the pressure is decreased on an equilibrium mixture of these three substances, equilibrium will ____, because ____.
A) shift to the right; lower pressure favors fewer moles of gas
B) shift to the right; lower pressure favors more moles of gas
C) shift to the left; lower pressure favors fewer moles of gas
D) shift to the left; lower pressure favors more moles of gas
E) be unchanged; solid NH4Cl does not appear in the equilibrium constant expression.
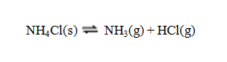 If the pressure is decreased on an equilibrium mixture of these three substances, equilibrium will ____, because ____.
If the pressure is decreased on an equilibrium mixture of these three substances, equilibrium will ____, because ____.A) shift to the right; lower pressure favors fewer moles of gas
B) shift to the right; lower pressure favors more moles of gas
C) shift to the left; lower pressure favors fewer moles of gas
D) shift to the left; lower pressure favors more moles of gas
E) be unchanged; solid NH4Cl does not appear in the equilibrium constant expression.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the exothermic reaction at equilibrium: 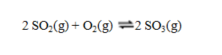 If the system is cooled, the equilibrium will ____, because ____.
If the system is cooled, the equilibrium will ____, because ____.
A) be unchanged; temperature has no effect on equilibrium
B) shift to the left; decreased temperature favors an exothermic reaction
C) shift to the right; decreased temperature favors an exothermic reaction
D) shift to the right; decreased temperature favors an endothermic reaction
E) shift to the left; decreased temperature favors an endothermic reaction
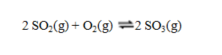 If the system is cooled, the equilibrium will ____, because ____.
If the system is cooled, the equilibrium will ____, because ____.A) be unchanged; temperature has no effect on equilibrium
B) shift to the left; decreased temperature favors an exothermic reaction
C) shift to the right; decreased temperature favors an exothermic reaction
D) shift to the right; decreased temperature favors an endothermic reaction
E) shift to the left; decreased temperature favors an endothermic reaction

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
41
If the value of Kc for a given reaction at a given temperature is a large number, the energy of the products is likely to be ____ the energy of the reactants because ____.
A) greater than; the value of Kc favors the reactants
B) less than; the value of Kc favors the reactants
C) the same as; the value of Kc favors neither the products nor the reactants
D) greater than; the value of Kc favors the products
E) less than; the value of Kc favors the products
A) greater than; the value of Kc favors the reactants
B) less than; the value of Kc favors the reactants
C) the same as; the value of Kc favors neither the products nor the reactants
D) greater than; the value of Kc favors the products
E) less than; the value of Kc favors the products

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
42
This reaction is the basis for the Haber-Bosch process for the manufacture of ammonia: 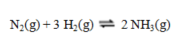 To maximize the yield of ammonia, the pressure ____ and the temperature____.
To maximize the yield of ammonia, the pressure ____ and the temperature____.
A) should be kept high by compressing the mixture; should be kept low
B) should be kept high by compressing the mixture; should be kept high
C) should be kept high by adding an inert gas; should be kept low
D) should be kept high by adding an inert gas; should be kept high
E) should be kept low; should be kept low
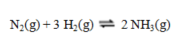 To maximize the yield of ammonia, the pressure ____ and the temperature____.
To maximize the yield of ammonia, the pressure ____ and the temperature____.A) should be kept high by compressing the mixture; should be kept low
B) should be kept high by compressing the mixture; should be kept high
C) should be kept high by adding an inert gas; should be kept low
D) should be kept high by adding an inert gas; should be kept high
E) should be kept low; should be kept low

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
43
The reaction
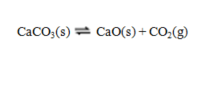 is the basis of a very ancient chemical industry. Seashells or chalk (limestone) are roasted in kilns to produce CaO (quicklime). When moistened and used in a mortar, this forms Ca(OH)2 (slaked lime). Atmospheric CO2 slowly converts this back to CaCO3. A strong bond thus results between the stones used in the wall.
is the basis of a very ancient chemical industry. Seashells or chalk (limestone) are roasted in kilns to produce CaO (quicklime). When moistened and used in a mortar, this forms Ca(OH)2 (slaked lime). Atmospheric CO2 slowly converts this back to CaCO3. A strong bond thus results between the stones used in the wall.
a. Is the above reaction exothermic or endothermic? Explain.
b. Lime kilns, since ancient times, have been designed with efficient chimneys to draw exhaust gases away. In terms of Le Chatelier's Principle, give two reasons why this enhances the conversion of limestone to quicklime.
c. When a sample of CaCO3(s) is placed in a sealed, evacuated container, the equilibrium CO2 pressure at a given temperature is always the same, and is not influenced by the amount of CaCO3, provided that there is still some present at equilibrium. Explain why.
d. How would the result in Part c be affected by adding an equal number of moles of CaO to the flask? Explain.
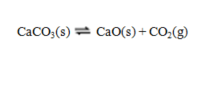 is the basis of a very ancient chemical industry. Seashells or chalk (limestone) are roasted in kilns to produce CaO (quicklime). When moistened and used in a mortar, this forms Ca(OH)2 (slaked lime). Atmospheric CO2 slowly converts this back to CaCO3. A strong bond thus results between the stones used in the wall.
is the basis of a very ancient chemical industry. Seashells or chalk (limestone) are roasted in kilns to produce CaO (quicklime). When moistened and used in a mortar, this forms Ca(OH)2 (slaked lime). Atmospheric CO2 slowly converts this back to CaCO3. A strong bond thus results between the stones used in the wall.a. Is the above reaction exothermic or endothermic? Explain.
b. Lime kilns, since ancient times, have been designed with efficient chimneys to draw exhaust gases away. In terms of Le Chatelier's Principle, give two reasons why this enhances the conversion of limestone to quicklime.
c. When a sample of CaCO3(s) is placed in a sealed, evacuated container, the equilibrium CO2 pressure at a given temperature is always the same, and is not influenced by the amount of CaCO3, provided that there is still some present at equilibrium. Explain why.
d. How would the result in Part c be affected by adding an equal number of moles of CaO to the flask? Explain.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
44
For an exothermic reaction, an increase in temperature
A) always means an increase in Kc
B) always means a decrease in Kc
C) the reaction will become more product-favored at higher temperatures
D) the reaction will become more reactant-favored at higher temperatures
E) both b and d
A) always means an increase in Kc
B) always means a decrease in Kc
C) the reaction will become more product-favored at higher temperatures
D) the reaction will become more reactant-favored at higher temperatures
E) both b and d

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
45
If a reaction is product-favored on the basis of enthalpy and reactant-favored on the basis of entropy, then it most likely is ____ and ____ net moles of gas.
A) exothermic; produces
B) exothermic; consumes
C) exothermic; neither produces nor consumes
D) endothermic; produces
E) endothermic; consumes
A) exothermic; produces
B) exothermic; consumes
C) exothermic; neither produces nor consumes
D) endothermic; produces
E) endothermic; consumes

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the reaction
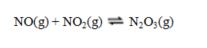 for which Kp = 2.00 at 20 C. A mixture of NO(g) at a partial pressure of 1.50 atm and NO2(g) at a partial pressure of 0.50 atm is allowed to come to equilibrium in a sealed container maintained at 20 C. What is the total pressure now?
for which Kp = 2.00 at 20 C. A mixture of NO(g) at a partial pressure of 1.50 atm and NO2(g) at a partial pressure of 0.50 atm is allowed to come to equilibrium in a sealed container maintained at 20 C. What is the total pressure now?
The expression for
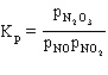 .
.
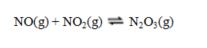 for which Kp = 2.00 at 20 C. A mixture of NO(g) at a partial pressure of 1.50 atm and NO2(g) at a partial pressure of 0.50 atm is allowed to come to equilibrium in a sealed container maintained at 20 C. What is the total pressure now?
for which Kp = 2.00 at 20 C. A mixture of NO(g) at a partial pressure of 1.50 atm and NO2(g) at a partial pressure of 0.50 atm is allowed to come to equilibrium in a sealed container maintained at 20 C. What is the total pressure now?The expression for
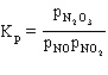 .
. 
Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
47
Which statement concerning product-favored reactions is not correct?
A) If a reaction is product-favored at high temperature, the entropy of the products is probably greater than the entropy of the reactants.
B) If a reaction is product-favored at low temperature, the enthalpy of the products is probably less than the enthalpy of the reactants.
C) If the entropy of the products is greater than the entropy of the reactants, the reaction is product-favored.
D) An endothermic reaction is product-favored.
E) The value of the equilibrium constant is greater than 1.
A) If a reaction is product-favored at high temperature, the entropy of the products is probably greater than the entropy of the reactants.
B) If a reaction is product-favored at low temperature, the enthalpy of the products is probably less than the enthalpy of the reactants.
C) If the entropy of the products is greater than the entropy of the reactants, the reaction is product-favored.
D) An endothermic reaction is product-favored.
E) The value of the equilibrium constant is greater than 1.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is true for an equilibrium system?
A) in an equilibrium system, molecules that are higher in energy occur less often
B) if there are more product molecules than reactant molecules, entropy favors the products in an equilibrium system
C) the higher the temperature is, the less important the energy effect (DH) becomes
D) the higher the temperature is, the more the entropy effect (dispersal of energy) determines the position of equilibrium
E) all of these
A) in an equilibrium system, molecules that are higher in energy occur less often
B) if there are more product molecules than reactant molecules, entropy favors the products in an equilibrium system
C) the higher the temperature is, the less important the energy effect (DH) becomes
D) the higher the temperature is, the more the entropy effect (dispersal of energy) determines the position of equilibrium
E) all of these

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
49
Consider the statement, "At equilibrium, a reaction has proceeded as far as it will go, and no further reaction will be observed."
a. From a macroscopic viewpoint, is this statement correct ? Explain your answer.
b. From a nanoscale viewpoint, is this statement correct? Explain your answer.
a. From a macroscopic viewpoint, is this statement correct ? Explain your answer.
b. From a nanoscale viewpoint, is this statement correct? Explain your answer.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
50
In predicting which side of an equilibrium is more favored, the factor concerned with probability is called
A) enthalpy.
B) endoscopy.
C) endothermic.
D) entropy.
E) exothermic.
A) enthalpy.
B) endoscopy.
C) endothermic.
D) entropy.
E) exothermic.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the reaction
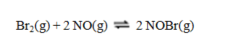 for which
for which
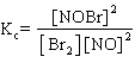 .
.
A sample of pure NOBr is isolated at low temperature. It is placed in a flask at a concentration of 0.200 M and warmed up to 50 C. When the reaction has come to equilibrium, the concentration of NOBr is 0.176 M. What is the value of Kc at 50 C for this reaction?
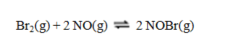 for which
for which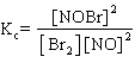 .
.A sample of pure NOBr is isolated at low temperature. It is placed in a flask at a concentration of 0.200 M and warmed up to 50 C. When the reaction has come to equilibrium, the concentration of NOBr is 0.176 M. What is the value of Kc at 50 C for this reaction?

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
52
For a particular chemical reaction, which of the following will result in a change of the equilibrium constant?
A) a change in pressure
B) a change in the temperature
C) adding additional reactant
D) removing product
E) the equilibrium constant never changes
A) a change in pressure
B) a change in the temperature
C) adding additional reactant
D) removing product
E) the equilibrium constant never changes

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
53
Considering only the probability factor in the gas-phase reaction C  2 A + B, ____ side is favored because ____.
2 A + B, ____ side is favored because ____.
A) the product; there are more possible arrangements of molecules on the reactant side
B) the product; there are more possible arrangements of molecules on the product side
C) the reactant; there are more possible arrangements of molecules on the reactant side
D) the reactant; there are more possible arrangements of molecules on the product side
E) one cannot say which; more information is needed.
 2 A + B, ____ side is favored because ____.
2 A + B, ____ side is favored because ____.A) the product; there are more possible arrangements of molecules on the reactant side
B) the product; there are more possible arrangements of molecules on the product side
C) the reactant; there are more possible arrangements of molecules on the reactant side
D) the reactant; there are more possible arrangements of molecules on the product side
E) one cannot say which; more information is needed.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
54
Consider the reaction 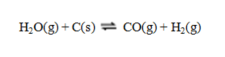 Consider an equilibrium mixture of these substances. If the pressure is increased by compressing the mixture, equilibrium will ____; if pressure is increased by adding an inert gas to the mixture, equilibrium will ____.
Consider an equilibrium mixture of these substances. If the pressure is increased by compressing the mixture, equilibrium will ____; if pressure is increased by adding an inert gas to the mixture, equilibrium will ____.
A) shift to the left; shift to the left
B) shift to the right; shift to the left
C) shift to the left; remain unchanged
D) shift to the right; remain unchanged
E) shift to the right; shift to the right
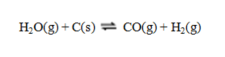 Consider an equilibrium mixture of these substances. If the pressure is increased by compressing the mixture, equilibrium will ____; if pressure is increased by adding an inert gas to the mixture, equilibrium will ____.
Consider an equilibrium mixture of these substances. If the pressure is increased by compressing the mixture, equilibrium will ____; if pressure is increased by adding an inert gas to the mixture, equilibrium will ____.A) shift to the left; shift to the left
B) shift to the right; shift to the left
C) shift to the left; remain unchanged
D) shift to the right; remain unchanged
E) shift to the right; shift to the right

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
55
As the temperature is increased, which kinds of reactions become more product-favored?
A) Only those that are endothermic and result in an increase in entropy.
B) Only those that are exothermic and result in an increase in entropy.
C) All reactions that result in an increase in entropy.
D) All reactions that result in an decrease in entropy.
E) Only those that are exothermic and result in an decrease in entropy.
A) Only those that are endothermic and result in an increase in entropy.
B) Only those that are exothermic and result in an increase in entropy.
C) All reactions that result in an increase in entropy.
D) All reactions that result in an decrease in entropy.
E) Only those that are exothermic and result in an decrease in entropy.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
56
If the value of Kc for a given reaction at a given temperature is a small number, the energy of the products is likely to be ____ the energy of the reactants because ____.
A) greater than; the value of Kc favors the reactants
B) less than; the value of Kc favors the reactants
C) the same as; the value of Kc favors neither the products nor the reactants
D) greater than; the value of Kc favors the products
E) less than; the value of Kc favors the products
A) greater than; the value of Kc favors the reactants
B) less than; the value of Kc favors the reactants
C) the same as; the value of Kc favors neither the products nor the reactants
D) greater than; the value of Kc favors the products
E) less than; the value of Kc favors the products

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
57
Concerning the Haber-Bosch for the synthesis of ammonia, 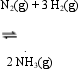 which of the following is true?
which of the following is true?
A) the reaction is strongly exothermic
B) the reaction is carried out at high pressure
C) ammonia is continually liquefied and removed from the process
D) the temperature is raised because the reaction is too slow at room temperature
E) all of these
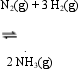 which of the following is true?
which of the following is true?A) the reaction is strongly exothermic
B) the reaction is carried out at high pressure
C) ammonia is continually liquefied and removed from the process
D) the temperature is raised because the reaction is too slow at room temperature
E) all of these

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck


