Exam 13: Chemical Equilibrium
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
Consider the statement, "At equilibrium, a reaction has proceeded as far as it will go, and no further reaction will be observed."
a. From a macroscopic viewpoint, is this statement correct ? Explain your answer.
b. From a nanoscale viewpoint, is this statement correct? Explain your answer.
Free
(Essay)
4.7/5  (35)
(35)
Correct Answer:
a. Partially. It is right that the system will be apparently unchanging to a casual inspection once equilibrium is attained. However, macroscopic observation cannot determine if reaction has proceeded "as far as it will go"-which implies that it has stopped. Infact, it has not.
b. No. The reaction (in both directions) wil be observed to continue. However the rates in the two directions are equal, which explains the macroscopic observation of Part a.
Consider the reaction ![Consider the reaction At equilibrium, [CO] = 4.14 × 10<sup>-6</sup> M; [Cl<sub>2</sub>] = 4.14 × 10<sup>-6</sup> M; and [COCl<sub>2</sub>] = 0.0627 M. Calculate the value of the equilibrium constant, K<sub>c</sub>.](https://storage.examlex.com/TB5061/11ec6ef9_ba27_6a04_8fe2_8d74aeeb9d71_TB5061_00.jpg) At equilibrium, [CO] = 4.14 × 10-6 M; [Cl2] = 4.14 × 10-6 M; and [COCl2] = 0.0627 M. Calculate the value of the equilibrium constant, Kc.
At equilibrium, [CO] = 4.14 × 10-6 M; [Cl2] = 4.14 × 10-6 M; and [COCl2] = 0.0627 M. Calculate the value of the equilibrium constant, Kc.
Free
(Multiple Choice)
4.8/5  (26)
(26)
Correct Answer:
A
Consider the reaction 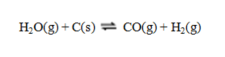 Consider an equilibrium mixture of these substances. If the pressure is increased by compressing the mixture, equilibrium will ____; if pressure is increased by adding an inert gas to the mixture, equilibrium will ____.
Consider an equilibrium mixture of these substances. If the pressure is increased by compressing the mixture, equilibrium will ____; if pressure is increased by adding an inert gas to the mixture, equilibrium will ____.
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
C
As the temperature is increased, which kinds of reactions become more product-favored?
(Multiple Choice)
4.7/5  (45)
(45)
An endothermic reaction which results in an increase in moles of gas will be most product-favored under conditions of ____ pressure and ____ temperature.
(Multiple Choice)
4.8/5  (34)
(34)
If the value of Kc for a given reaction at a given temperature is a small number, the energy of the products is likely to be ____ the energy of the reactants because ____.
(Multiple Choice)
4.9/5  (39)
(39)
To decide whether a reaction mixture is at equilibrium, a student determines the value of Q, the reaction quotient, and finds that it is less than K. Therefore, the mixture is
(Multiple Choice)
4.8/5  (43)
(43)
Terms relating to certain types of substances are conventionally omitted from equilibrium constant expressions. These substances are
(Multiple Choice)
4.8/5  (31)
(31)
Consider the equilibrium reaction 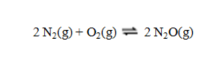 In a particular experiment, the equilibrium concentration of N2 is 0.048 M, of O2, 0.093 M, and of N2O, 6.55 × 10-21 M. What is the value of the equilibrium constant Kc?
In a particular experiment, the equilibrium concentration of N2 is 0.048 M, of O2, 0.093 M, and of N2O, 6.55 × 10-21 M. What is the value of the equilibrium constant Kc?
(Multiple Choice)
4.8/5  (26)
(26)
The equilibrium constant for the reaction 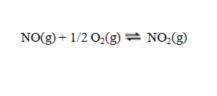 has a value of Kc = 1.23 at a certain temperature. What is the value of Kc for the reaction:
has a value of Kc = 1.23 at a certain temperature. What is the value of Kc for the reaction:
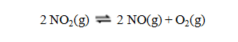
(Multiple Choice)
4.7/5  (40)
(40)
Consider the exothermic reaction at equilibrium: 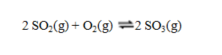 If the system is cooled, the equilibrium will ____, because ____.
If the system is cooled, the equilibrium will ____, because ____.
(Multiple Choice)
4.8/5  (34)
(34)
Consider the reaction 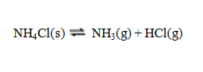 If an equilibrium mixture of these three substances is compressed, equilibrium will ____, because ____.
If an equilibrium mixture of these three substances is compressed, equilibrium will ____, because ____.
(Multiple Choice)
4.8/5  (35)
(35)
Considering only the probability factor in the gas-phase reaction C  2 A + B, ____ side is favored because ____.
2 A + B, ____ side is favored because ____.
(Multiple Choice)
4.8/5  (30)
(30)
The concentration equilibrium constant, Kc, and the pressure equilibrium constant, Kp, are related by the expression
(Multiple Choice)
4.9/5  (43)
(43)
In predicting which side of an equilibrium is more favored, the factor concerned with probability is called
(Multiple Choice)
4.8/5  (34)
(34)
Consider the reaction 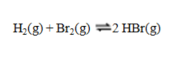 If the partial pressures in an equilibrium mixture of H2, Br2, and HBr are 0.024 atm, 0.031 atm, and 5.07 atm, respectively, the value of Kp for this reaction at this temperature is
If the partial pressures in an equilibrium mixture of H2, Br2, and HBr are 0.024 atm, 0.031 atm, and 5.07 atm, respectively, the value of Kp for this reaction at this temperature is
(Multiple Choice)
4.8/5  (24)
(24)
Consider the endothermic reaction 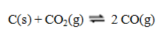 If such a system at equilibrium is heated, equilibrium will ____, because ____.
If such a system at equilibrium is heated, equilibrium will ____, because ____.
(Multiple Choice)
4.8/5  (39)
(39)
Consider the equilibrium system 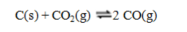 If more C(s) is added, the equilibrium will ____; if CO is removed the equilibrium will ____.
If more C(s) is added, the equilibrium will ____; if CO is removed the equilibrium will ____.
(Multiple Choice)
5.0/5  (36)
(36)
The equilibrium constant expression for the reaction shown below is 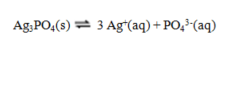
(Multiple Choice)
4.8/5  (29)
(29)
The equilibrium constant for a reaction is related to the rate constants for the reaction as follows:
(Multiple Choice)
4.9/5  (25)
(25)
Showing 1 - 20 of 57
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)