Deck 12: Chemical Kinetics: Rates of Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/66
Play
Full screen (f)
Deck 12: Chemical Kinetics: Rates of Reactions
1
Exhibit 12-1 The following question(s) relate to the reaction between water and a complex ion of Co2+; the rate varies with concentration as follows. (The rate has no dependence on [H2O].)
![<strong>Exhibit 12-1 The following question(s) relate to the reaction between water and a complex ion of Co<sup>2+</sup>; the rate varies with concentration as follows. (The rate has no dependence on [H<sub>2</sub>O].) Refer to Exhibit 12-1. The rate law for the reaction is rate =</strong> A) k<sup>2</sup>[Co<sup>2+</sup> complex]. B) k[Co<sup>2+</sup> complex]. C) 2k[Co<sup>2+</sup> complex]. D) k[Co<sup>2+</sup> complex]/2. E) k[Co<sup>2+</sup> complex]<sup>2</sup>.](https://storage.examlex.com/TB5061/11ea782d_fd82_0705_bbd5_a3012719dd12_TB5061_00.jpg) Refer to Exhibit 12-1. The rate law for the reaction is rate =
Refer to Exhibit 12-1. The rate law for the reaction is rate =
A) k2[Co2+ complex].
B) k[Co2+ complex].
C) 2k[Co2+ complex].
D) k[Co2+ complex]/2.
E) k[Co2+ complex]2.
![<strong>Exhibit 12-1 The following question(s) relate to the reaction between water and a complex ion of Co<sup>2+</sup>; the rate varies with concentration as follows. (The rate has no dependence on [H<sub>2</sub>O].) Refer to Exhibit 12-1. The rate law for the reaction is rate =</strong> A) k<sup>2</sup>[Co<sup>2+</sup> complex]. B) k[Co<sup>2+</sup> complex]. C) 2k[Co<sup>2+</sup> complex]. D) k[Co<sup>2+</sup> complex]/2. E) k[Co<sup>2+</sup> complex]<sup>2</sup>.](https://storage.examlex.com/TB5061/11ea782d_fd82_0705_bbd5_a3012719dd12_TB5061_00.jpg) Refer to Exhibit 12-1. The rate law for the reaction is rate =
Refer to Exhibit 12-1. The rate law for the reaction is rate =A) k2[Co2+ complex].
B) k[Co2+ complex].
C) 2k[Co2+ complex].
D) k[Co2+ complex]/2.
E) k[Co2+ complex]2.
k[Co2+ complex].
2
Exhibit 12-1 The following question(s) relate to the reaction between water and a complex ion of Co2+; the rate varies with concentration as follows. (The rate has no dependence on [H2O].)
![<strong>Exhibit 12-1 The following question(s) relate to the reaction between water and a complex ion of Co<sup>2+</sup>; the rate varies with concentration as follows. (The rate has no dependence on [H<sub>2</sub>O].) Refer to Exhibit 12-1. The value and units of the rate constant are:</strong> A) 3.6 × 10<sup>3</sup> M min<sup>-1</sup>. B) 2.8 × 10<sup>-4</sup> M min<sup>-1</sup>. C) 3.6 × 10<sup>3</sup> min<sup>-1</sup>. D) 2.8 × 10<sup>-4</sup> min<sup>-1</sup>. E) 3.6 × 10<sup>3</sup> M<sup>-1</sup> min<sup>-1</sup>.](https://storage.examlex.com/TB5061/11ea782d_fd82_2e16_bbd5_37d006af06ca_TB5061_00.jpg) Refer to Exhibit 12-1. The value and units of the rate constant are:
Refer to Exhibit 12-1. The value and units of the rate constant are:
A) 3.6 × 103 M min-1.
B) 2.8 × 10-4 M min-1.
C) 3.6 × 103 min-1.
D) 2.8 × 10-4 min-1.
E) 3.6 × 103 M-1 min-1.
![<strong>Exhibit 12-1 The following question(s) relate to the reaction between water and a complex ion of Co<sup>2+</sup>; the rate varies with concentration as follows. (The rate has no dependence on [H<sub>2</sub>O].) Refer to Exhibit 12-1. The value and units of the rate constant are:</strong> A) 3.6 × 10<sup>3</sup> M min<sup>-1</sup>. B) 2.8 × 10<sup>-4</sup> M min<sup>-1</sup>. C) 3.6 × 10<sup>3</sup> min<sup>-1</sup>. D) 2.8 × 10<sup>-4</sup> min<sup>-1</sup>. E) 3.6 × 10<sup>3</sup> M<sup>-1</sup> min<sup>-1</sup>.](https://storage.examlex.com/TB5061/11ea782d_fd82_2e16_bbd5_37d006af06ca_TB5061_00.jpg) Refer to Exhibit 12-1. The value and units of the rate constant are:
Refer to Exhibit 12-1. The value and units of the rate constant are:A) 3.6 × 103 M min-1.
B) 2.8 × 10-4 M min-1.
C) 3.6 × 103 min-1.
D) 2.8 × 10-4 min-1.
E) 3.6 × 103 M-1 min-1.
2.8 × 10-4 min-1.
3
Which of the following factors will affect the rate of a homogeneous reaction?
A) the presence of a catalyst, and, if one is present, its concentration
B) the properties (particularly, molecular structure and bonding) of reactants and products
C) the concentrations of the reactants and sometimes the products
D) the temperature at which the reaction occurs
E) all of these
A) the presence of a catalyst, and, if one is present, its concentration
B) the properties (particularly, molecular structure and bonding) of reactants and products
C) the concentrations of the reactants and sometimes the products
D) the temperature at which the reaction occurs
E) all of these
all of these
4
For many homogeneous reactions, the rate law has the general form
A) Rate = 1/k[A]m[B]n
B) Rate = k[A]m[B]n
C) Rate = k[A]1/m[B]1/n
D) Rate = k[1/A]m[1/B]n
E) None of these
A) Rate = 1/k[A]m[B]n
B) Rate = k[A]m[B]n
C) Rate = k[A]1/m[B]1/n
D) Rate = k[1/A]m[1/B]n
E) None of these

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
5
Which unit is appropriate for describing reaction rates?
A) kJ mol-1
B) ( C) min-1
C) M min-1
D) kJ g-1
E) g L-1
A) kJ mol-1
B) ( C) min-1
C) M min-1
D) kJ g-1
E) g L-1

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
6
The units for the rate constant k must always include
A) a volume unit
B) a concentration unit
C) a time unit
D) all of these
E) none of these
A) a volume unit
B) a concentration unit
C) a time unit
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
7
Which statement about a rate constant is correct?
A) Its units are always M min-1.
B) Its value generally decreases as temperature increases.
C) Its value depends on the concentration of the reactant(s).
D) Its value at a particular temperature depends on the reaction involved.
E) It is symbolized by "K".
A) Its units are always M min-1.
B) Its value generally decreases as temperature increases.
C) Its value depends on the concentration of the reactant(s).
D) Its value at a particular temperature depends on the reaction involved.
E) It is symbolized by "K".

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
8
At a given temperature, the kinetics parameters for a new chemical reaction are determined in the following sequence from first to last
A) orders of reaction, k, rate law
B) k, orders of reaction, rate law
C) orders of reaction, rate law, k
D) k, rate law, orders of reaction
E) rate law, k, orders of reaction
A) orders of reaction, k, rate law
B) k, orders of reaction, rate law
C) orders of reaction, rate law, k
D) k, rate law, orders of reaction
E) rate law, k, orders of reaction

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
9
The rate of the chemical reaction involving two substances, A and B, is measured. It is found that if the initial concentration of A used is doubled, keeping the B concentration the same, the rate doubles. If the concentrations of both A and B are doubled, the rate is eight times that measured in the first experiment. The rate law for this reaction is rate =
A) k[A][B].
B) k[A]2[B].
C) k[A][B]2.
D) 2k[A][B].
E) k[A][B]/2.
A) k[A][B].
B) k[A]2[B].
C) k[A][B]2.
D) 2k[A][B].
E) k[A][B]/2.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
10
A heterogeneous reaction mixture may contain
A) only a solid phase and a liquid phase
B) only a gas phase and a solid phase
C) only a gas phase and a liquid phase
D) a gas phase, a solid phase, and a liquid phase
E) any of these
A) only a solid phase and a liquid phase
B) only a gas phase and a solid phase
C) only a gas phase and a liquid phase
D) a gas phase, a solid phase, and a liquid phase
E) any of these

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following could not be the units of a rate constant?
A) min-1
B) M min-1
C) M-1 min-1
D) M2 min-1
E) M-2 min-1
A) min-1
B) M min-1
C) M-1 min-1
D) M2 min-1
E) M-2 min-1

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
12
In a reaction that is first-order with respect to reactant A and second-order with respect to reactant B, the rate of the reaction
A) doubles with a doubling of the concentration of either reactant
B) doubles with a doubling of the concentration of B
C) is halved by a doubling of the concentration of B
D) is tripled by any equal and simultaneous increase in the concentration of A and B
E) quadrupled by a doubling in the concentration of B while the concentration of A is held constant
A) doubles with a doubling of the concentration of either reactant
B) doubles with a doubling of the concentration of B
C) is halved by a doubling of the concentration of B
D) is tripled by any equal and simultaneous increase in the concentration of A and B
E) quadrupled by a doubling in the concentration of B while the concentration of A is held constant

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the hypothetical reaction ![<strong>Consider the hypothetical reaction The rate of reaction is ____ times D[B]/Dt and ____ times D[C]/Dt.</strong> A) -2; -3 B) -1/3; 1/2 C) 1/2; -1/3 D) -1/2; -1/3 E) -1; 2/3](https://storage.examlex.com/TB5061/11ea782d_fd81_b8e4_bbd5_a5dd54ed6b53_TB5061_00.jpg) The rate of reaction is ____ times D[B]/Dt and ____ times D[C]/Dt.
The rate of reaction is ____ times D[B]/Dt and ____ times D[C]/Dt.
A) -2; -3
B) -1/3; 1/2
C) 1/2; -1/3
D) -1/2; -1/3
E) -1; 2/3
![<strong>Consider the hypothetical reaction The rate of reaction is ____ times D[B]/Dt and ____ times D[C]/Dt.</strong> A) -2; -3 B) -1/3; 1/2 C) 1/2; -1/3 D) -1/2; -1/3 E) -1; 2/3](https://storage.examlex.com/TB5061/11ea782d_fd81_b8e4_bbd5_a5dd54ed6b53_TB5061_00.jpg) The rate of reaction is ____ times D[B]/Dt and ____ times D[C]/Dt.
The rate of reaction is ____ times D[B]/Dt and ____ times D[C]/Dt.A) -2; -3
B) -1/3; 1/2
C) 1/2; -1/3
D) -1/2; -1/3
E) -1; 2/3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
14
The rate law for a given reaction is rate = k[reactant]2, with k = 2.64 × 10-4 M-1 min-1. If the initial concentration is 0.0250 M, what is the initial rate, with the correct units?
A) 4.36 × 10-11 M min-1
B) 1.65 × 10-7 min-1
C) 6.60 × 10-6 M min-1
D) 1.65 × 10-7 M min-1
E) 6.60 × 10-6 min-1
A) 4.36 × 10-11 M min-1
B) 1.65 × 10-7 min-1
C) 6.60 × 10-6 M min-1
D) 1.65 × 10-7 M min-1
E) 6.60 × 10-6 min-1

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
15
If a reaction is first-order with respect to each of its two reactants, then the rate of the reaction is
A) inversely proportional to the concentration of each reactant
B) directly proportional to the concentration of one reactant and inversely proportional to the concentration of the other reactant
C) directly proportional to the inverse of the concentration of each of the two reactants
D) unaffected by concentration as long as the concentrations of the two reactants are always equal to one another
E) directly proportional to the concentration of each reactant
A) inversely proportional to the concentration of each reactant
B) directly proportional to the concentration of one reactant and inversely proportional to the concentration of the other reactant
C) directly proportional to the inverse of the concentration of each of the two reactants
D) unaffected by concentration as long as the concentrations of the two reactants are always equal to one another
E) directly proportional to the concentration of each reactant

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
16
For a reaction that is zeroth-order with respect to reactant A, and nth-order overall
A) Rate = k[0]m[B]n
B) Rate = k[B]n
C) Rate = k[A]m[0]n
D) Rate = k[A]m[B]0
E) Rate = k[A]0[B]0
A) Rate = k[0]m[B]n
B) Rate = k[B]n
C) Rate = k[A]m[0]n
D) Rate = k[A]m[B]0
E) Rate = k[A]0[B]0

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
17
For a new chemical reaction involving several reactants, the order of reaction with respect to each reactant
A) is easily determined from reactions similar to the one in question
B) may be determined from the coefficient for the respective reactant in the balanced chemical equation
C) must be determined from the rate constant at time = 0
D) must be determined from the overall order of the reaction
E) must be determined experimentally using one or more methods
A) is easily determined from reactions similar to the one in question
B) may be determined from the coefficient for the respective reactant in the balanced chemical equation
C) must be determined from the rate constant at time = 0
D) must be determined from the overall order of the reaction
E) must be determined experimentally using one or more methods

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following units may be used for describing reaction rates?
A) mol L-1 s-1
B) M min-1
C) M h-1
D) M s-1
E) all of these
A) mol L-1 s-1
B) M min-1
C) M h-1
D) M s-1
E) all of these

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following factors will affect the rate of a reaction only if it is heterogeneous?
A) the surface area of the reactants
B) the concentrations of reactants
C) the concentrations of the products
D) the temperature of the reaction system
E) the presence of catalysts
A) the surface area of the reactants
B) the concentrations of reactants
C) the concentrations of the products
D) the temperature of the reaction system
E) the presence of catalysts

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
20
The rate of a chemical reaction may be expressed as
A) the instantaneous rate at some moment in time after the reaction has begun
B) the average change in the concentration of a reactant or product over some unit of time
C) the instantaneous rate at the very beginning of the reaction when time = 0
D) any of these
E) none of these
A) the instantaneous rate at some moment in time after the reaction has begun
B) the average change in the concentration of a reactant or product over some unit of time
C) the instantaneous rate at the very beginning of the reaction when time = 0
D) any of these
E) none of these

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
21
The decomposition of N2O(g) to nitrogen and oxygen has the rate law 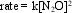 where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
A) 9.5 × 10-3 M
B) 2.2 × 10-3 M
C) 0.029 M
D) 0.17 M
E) 0.46 M
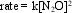 where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?A) 9.5 × 10-3 M
B) 2.2 × 10-3 M
C) 0.029 M
D) 0.17 M
E) 0.46 M

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
22
In a bimolecular reaction, which term can be equated to the probability that a collision between molecules will have the correct relative orientation for chemical reaction to occur?
A) steric factor
B) Arrhenius Equation
C) molecularity
D) activation energy
E) frequency factor
A) steric factor
B) Arrhenius Equation
C) molecularity
D) activation energy
E) frequency factor

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
23
A certain reaction is studied at room temperature, and gives a straight line plot for 1/[reactant] versus time. Which one of the following statements is true?
A) The same reaction at a higher temperature would yield a similar plot parallel to the original graph, with a larger value for the y-intercept.
B) The same reaction at a higher temperature would yield a similar plot parallel to the original graph, with a smaller value for the y-intercept.
C) The reaction is second-order.
D) The same reaction at a higher temperature would yield a plot with a smaller slope than the original graph, but with the same y-intercept.
E) A graph of ln[reactant] versus 1/time will be a straight line at both temperatures.
A) The same reaction at a higher temperature would yield a similar plot parallel to the original graph, with a larger value for the y-intercept.
B) The same reaction at a higher temperature would yield a similar plot parallel to the original graph, with a smaller value for the y-intercept.
C) The reaction is second-order.
D) The same reaction at a higher temperature would yield a plot with a smaller slope than the original graph, but with the same y-intercept.
E) A graph of ln[reactant] versus 1/time will be a straight line at both temperatures.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
24
From the stoichiometry of the reaction 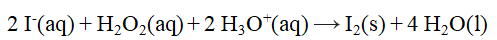 the rate law
the rate law
A) is predicted to be third-order overall.
B) is predicted to be first-order in I2(s).
C) is predicted to be first-order in all reactants.
D) is predicted to be fifth-order overall.
E) cannot be predicted.
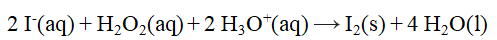 the rate law
the rate lawA) is predicted to be third-order overall.
B) is predicted to be first-order in I2(s).
C) is predicted to be first-order in all reactants.
D) is predicted to be fifth-order overall.
E) cannot be predicted.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
25
The Arrhenius equation is
A)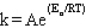
B)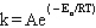
C)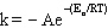
D)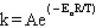
E)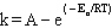
A)
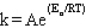
B)
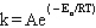
C)
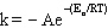
D)
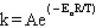
E)
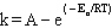

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
26
The half-life for the first-order conversion of A to B is 2.22 hr. What is the rate constant?
A) 0.312 hr-1
B) 0.465 hr-1
C) 1.54 hr-1
D) 2.22 hr-1
E) 3.20 hr-1
A) 0.312 hr-1
B) 0.465 hr-1
C) 1.54 hr-1
D) 2.22 hr-1
E) 3.20 hr-1

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
27
The species representing the combination of atoms or molecules at the top of the activation energy barrier is called the
A) frequency factor.
B) activated complex.
C) catalytic convertor.
D) reaction intermediate.
E) enthalpy of reaction.
A) frequency factor.
B) activated complex.
C) catalytic convertor.
D) reaction intermediate.
E) enthalpy of reaction.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
28
For a first-order reaction, which expression describes the relationship between the rate constant, k, and the half-life, t1/2?
A) t1/2 = 2 / k
B) t1/2 = ln 2 / k
C) t1/2 = k / 2
D) t1/2 = ln 2 × k
E) t1/2 = k / ln 2
A) t1/2 = 2 / k
B) t1/2 = ln 2 / k
C) t1/2 = k / 2
D) t1/2 = ln 2 × k
E) t1/2 = k / ln 2

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
29
A reaction displays zero-order kinetics for its single reactant. It therefore follows that a plot of ____ versus time is linear, and that the slope of this plot = ____.
A) [reactant]; -k
B) [reactant]; k
C) 1/[reactant]; -k
D) 1/[reactant]; k
E) ln[reactant]; -k
A) [reactant]; -k
B) [reactant]; k
C) 1/[reactant]; -k
D) 1/[reactant]; k
E) ln[reactant]; -k

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
30
The frequency factor in the Arrhenius equation can best be described as
A) the probability that a collision between molecules will have the correct relative orientation for reaction to occur.
B) a numerical description of the amount of energy released by colliding reactant molecules when they form products.
C) a numerical description of how often molecules collide with the proper orientation to react at a specific concentration.
D) a numerical description of the amount of energy needed by colliding reactant molecules in order to form products.
E) a factor which corrects for the conversion between J and kJ.
A) the probability that a collision between molecules will have the correct relative orientation for reaction to occur.
B) a numerical description of the amount of energy released by colliding reactant molecules when they form products.
C) a numerical description of how often molecules collide with the proper orientation to react at a specific concentration.
D) a numerical description of the amount of energy needed by colliding reactant molecules in order to form products.
E) a factor which corrects for the conversion between J and kJ.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
31
Consider the reaction with the mechanism shown:  The first step is ____; the second step is ____; and the third step is ____.
The first step is ____; the second step is ____; and the third step is ____.
A) unimolecular; unimolecular; unimolecular
B) unimolecular; bimolecular; bimolecular
C) bimolecular; unimolecular; bimolecular
D) bimolecular; bimolecular; unimolecular
E) bimolecular; bimolecular; bimolecular
 The first step is ____; the second step is ____; and the third step is ____.
The first step is ____; the second step is ____; and the third step is ____.A) unimolecular; unimolecular; unimolecular
B) unimolecular; bimolecular; bimolecular
C) bimolecular; unimolecular; bimolecular
D) bimolecular; bimolecular; unimolecular
E) bimolecular; bimolecular; bimolecular

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the reaction ![<strong>Consider the reaction Use the data shown to determine the rate law for the reaction. </strong> A) rate = k[NO<sub>2</sub>] [O<sub>3</sub>] B) rate = k[NO<sub>2</sub>]<sup>2</sup> [O<sub>3</sub>] C) rate = k[NO<sub>2</sub>] [O<sub>3</sub>]<sup>2</sup> D) rate = k[NO<sub>2</sub>] E) rate = k[NO<sub>3</sub>] [O<sub>3</sub>] / [NO<sub>2</sub>] [O<sub>2</sub>]](https://storage.examlex.com/TB5061/11ec7128_e356_0add_88eb_ab607bfad5a6_TB5061_00.jpg) Use the data shown to determine the rate law for the reaction.
Use the data shown to determine the rate law for the reaction.
![<strong>Consider the reaction Use the data shown to determine the rate law for the reaction. </strong> A) rate = k[NO<sub>2</sub>] [O<sub>3</sub>] B) rate = k[NO<sub>2</sub>]<sup>2</sup> [O<sub>3</sub>] C) rate = k[NO<sub>2</sub>] [O<sub>3</sub>]<sup>2</sup> D) rate = k[NO<sub>2</sub>] E) rate = k[NO<sub>3</sub>] [O<sub>3</sub>] / [NO<sub>2</sub>] [O<sub>2</sub>]](https://storage.examlex.com/TB5061/11ea782d_fd82_ca5d_bbd5_8720949e1ab5_TB5061_00.jpg)
A) rate = k[NO2] [O3]
B) rate = k[NO2]2 [O3]
C) rate = k[NO2] [O3]2
D) rate = k[NO2]
E) rate = k[NO3] [O3] / [NO2] [O2]
![<strong>Consider the reaction Use the data shown to determine the rate law for the reaction. </strong> A) rate = k[NO<sub>2</sub>] [O<sub>3</sub>] B) rate = k[NO<sub>2</sub>]<sup>2</sup> [O<sub>3</sub>] C) rate = k[NO<sub>2</sub>] [O<sub>3</sub>]<sup>2</sup> D) rate = k[NO<sub>2</sub>] E) rate = k[NO<sub>3</sub>] [O<sub>3</sub>] / [NO<sub>2</sub>] [O<sub>2</sub>]](https://storage.examlex.com/TB5061/11ec7128_e356_0add_88eb_ab607bfad5a6_TB5061_00.jpg) Use the data shown to determine the rate law for the reaction.
Use the data shown to determine the rate law for the reaction.![<strong>Consider the reaction Use the data shown to determine the rate law for the reaction. </strong> A) rate = k[NO<sub>2</sub>] [O<sub>3</sub>] B) rate = k[NO<sub>2</sub>]<sup>2</sup> [O<sub>3</sub>] C) rate = k[NO<sub>2</sub>] [O<sub>3</sub>]<sup>2</sup> D) rate = k[NO<sub>2</sub>] E) rate = k[NO<sub>3</sub>] [O<sub>3</sub>] / [NO<sub>2</sub>] [O<sub>2</sub>]](https://storage.examlex.com/TB5061/11ea782d_fd82_ca5d_bbd5_8720949e1ab5_TB5061_00.jpg)
A) rate = k[NO2] [O3]
B) rate = k[NO2]2 [O3]
C) rate = k[NO2] [O3]2
D) rate = k[NO2]
E) rate = k[NO3] [O3] / [NO2] [O2]

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
33
The reaction 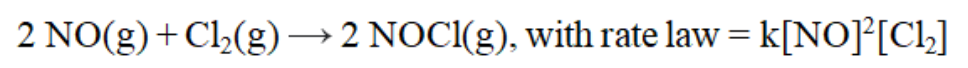 is ____ order with respect to NO, ____order with respect to Cl2, and ____ order overall.
is ____ order with respect to NO, ____order with respect to Cl2, and ____ order overall.
A) first; second; third
B) first; second; second
C) first; first; second
D) second; second; third
E) second; first; third
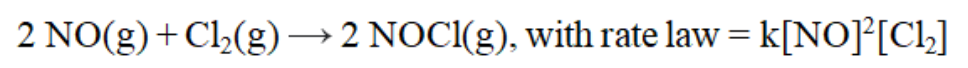 is ____ order with respect to NO, ____order with respect to Cl2, and ____ order overall.
is ____ order with respect to NO, ____order with respect to Cl2, and ____ order overall.A) first; second; third
B) first; second; second
C) first; first; second
D) second; second; third
E) second; first; third

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
34
The activation energy in the Arrhenius equation can best be described as
A) a numerical description of the amount of energy released by colliding reactant molecules when they form products.
B) a factor which corrects for the conversion between J and kJ.
C) a numerical description of how often molecules collide with the proper orientation to react at a specific concentration.
D) the probability that a collision between molecules will have the correct relative orientation for reaction to occur.
E) a numerical description of the amount of energy needed by colliding reactant molecules in order to form products.
A) a numerical description of the amount of energy released by colliding reactant molecules when they form products.
B) a factor which corrects for the conversion between J and kJ.
C) a numerical description of how often molecules collide with the proper orientation to react at a specific concentration.
D) the probability that a collision between molecules will have the correct relative orientation for reaction to occur.
E) a numerical description of the amount of energy needed by colliding reactant molecules in order to form products.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
35
If an elementary reaction is exothermic, then
A) The activation energy for the forward reaction is smaller than that for the reverse reaction.
B) The sum of activation energies gives the value of DH for the reaction.
C) The activation energy for the forward reaction is larger than that for the reverse reaction.
D) The activation energy for the forward reaction is positive, and for the reverse reaction, negative.
E) The activation energy for the forward reaction is negative, and for the reverse reaction, positive.
A) The activation energy for the forward reaction is smaller than that for the reverse reaction.
B) The sum of activation energies gives the value of DH for the reaction.
C) The activation energy for the forward reaction is larger than that for the reverse reaction.
D) The activation energy for the forward reaction is positive, and for the reverse reaction, negative.
E) The activation energy for the forward reaction is negative, and for the reverse reaction, positive.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
36
The rate constant for the first-order conversion of A to B is 3.33 hr-1. How much time will be required for the concentration of A to reach 75% of its original value?
A) 0.086 hr
B) 0.83 hr
C) 0.96 hr
D) 2.50 hr
E) 4.44 hr
A) 0.086 hr
B) 0.83 hr
C) 0.96 hr
D) 2.50 hr
E) 4.44 hr

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
37
For a zero-, first- or second-order reaction ![<strong>For a zero-, first- or second-order reaction the order of reaction with respect to reactant A may be determined</strong> A) from the specific rate law, if it is already known B) from the integrated rate law, if it is already known C) from plots of [A]<sub>t</sub> vs. t, ln[A]<sub>t</sub> vs. t, and 1/[A]<sub>t</sub> vs. t D) any of these E) none of these](https://storage.examlex.com/TB5061/11ea782d_fd82_7c38_bbd5_29f115d92b3e_TB5061_11.jpg) the order of reaction with respect to reactant A may be determined
the order of reaction with respect to reactant A may be determined
A) from the specific rate law, if it is already known
B) from the integrated rate law, if it is already known
C) from plots of [A]t vs. t, ln[A]t vs. t, and 1/[A]t vs. t
D) any of these
E) none of these
![<strong>For a zero-, first- or second-order reaction the order of reaction with respect to reactant A may be determined</strong> A) from the specific rate law, if it is already known B) from the integrated rate law, if it is already known C) from plots of [A]<sub>t</sub> vs. t, ln[A]<sub>t</sub> vs. t, and 1/[A]<sub>t</sub> vs. t D) any of these E) none of these](https://storage.examlex.com/TB5061/11ea782d_fd82_7c38_bbd5_29f115d92b3e_TB5061_11.jpg) the order of reaction with respect to reactant A may be determined
the order of reaction with respect to reactant A may be determinedA) from the specific rate law, if it is already known
B) from the integrated rate law, if it is already known
C) from plots of [A]t vs. t, ln[A]t vs. t, and 1/[A]t vs. t
D) any of these
E) none of these

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
38
When two atoms, molecules or ions are involved in an elementary reaction, it is said to be a(n):
A) activated complexation.
B) unimolecular reaction.
C) first-order reaction.
D) bimolecular reaction.
E) Arrhenius reaction.
A) activated complexation.
B) unimolecular reaction.
C) first-order reaction.
D) bimolecular reaction.
E) Arrhenius reaction.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
39
For the reaction ![<strong>For the reaction the integrated rate law for a particular order of reaction</strong> A) relates concentration [A]<sub>t</sub> to concentration [A]<sub>0</sub> B) allows the determination of k when [A]<sub>0</sub> and [A]<sub>t</sub> are known C) allows the calculation of [A]<sub>t</sub> when k and [A]<sub>0</sub> are known D) allows the calculation of [A]<sub>0</sub> when k and [A]<sub>t</sub> are known E) all of these](https://storage.examlex.com/TB5061/11ea782d_fd82_7c37_bbd5_f94cacc293d9_TB5061_00.jpg) the integrated rate law for a particular order of reaction
the integrated rate law for a particular order of reaction
A) relates concentration [A]t to concentration [A]0
B) allows the determination of k when [A]0 and [A]t are known
C) allows the calculation of [A]t when k and [A]0 are known
D) allows the calculation of [A]0 when k and [A]t are known
E) all of these
![<strong>For the reaction the integrated rate law for a particular order of reaction</strong> A) relates concentration [A]<sub>t</sub> to concentration [A]<sub>0</sub> B) allows the determination of k when [A]<sub>0</sub> and [A]<sub>t</sub> are known C) allows the calculation of [A]<sub>t</sub> when k and [A]<sub>0</sub> are known D) allows the calculation of [A]<sub>0</sub> when k and [A]<sub>t</sub> are known E) all of these](https://storage.examlex.com/TB5061/11ea782d_fd82_7c37_bbd5_f94cacc293d9_TB5061_00.jpg) the integrated rate law for a particular order of reaction
the integrated rate law for a particular order of reactionA) relates concentration [A]t to concentration [A]0
B) allows the determination of k when [A]0 and [A]t are known
C) allows the calculation of [A]t when k and [A]0 are known
D) allows the calculation of [A]0 when k and [A]t are known
E) all of these

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
40
The rate constant for the first order decomposition of SO2Cl2 is 1.37 × 10-3 min-1 at a certain temperature. If the concentration is initially 0.500 M, predict it after 300 min.
A) 6.85 × 10-4 M
B) 0.331 M
C) 0.500 M
D) 0.754 M
E) 1.33 M
A) 6.85 × 10-4 M
B) 0.331 M
C) 0.500 M
D) 0.754 M
E) 1.33 M

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
41
Exhibit 12-2 For the following question(s), consider the following reaction mechanism:
 Refer to Exhibit 12-2. The rate-limiting step in this reaction is Step ____, because ____.
Refer to Exhibit 12-2. The rate-limiting step in this reaction is Step ____, because ____.
A) 3; the last step is always the rate-limiting step
B) 2; it uses a product of the first step
C) 2; it is the slow step
D) 1; it is bimolecular
E) 1; the first step must occur before any others can occur
 Refer to Exhibit 12-2. The rate-limiting step in this reaction is Step ____, because ____.
Refer to Exhibit 12-2. The rate-limiting step in this reaction is Step ____, because ____.A) 3; the last step is always the rate-limiting step
B) 2; it uses a product of the first step
C) 2; it is the slow step
D) 1; it is bimolecular
E) 1; the first step must occur before any others can occur

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
42
The best description of the induced fit model of enzyme function is
A) a non substrate molecule must first bind to the active site, allowing the substrate to fit properly.
B) a molecule not directly involved in the catalysis interacts with a site on the protein far from the active site, changing the shape of the active site to make it work.
C) the active site, the substrate, or both change shape upon binding so that the activation energy of the desired change is decreased.
D) the active site has a definite, rigid shape that only fits one substrate molecule.
E) a short protein chain that blocks access to the active site is chemically removed as the substrate approaches.
A) a non substrate molecule must first bind to the active site, allowing the substrate to fit properly.
B) a molecule not directly involved in the catalysis interacts with a site on the protein far from the active site, changing the shape of the active site to make it work.
C) the active site, the substrate, or both change shape upon binding so that the activation energy of the desired change is decreased.
D) the active site has a definite, rigid shape that only fits one substrate molecule.
E) a short protein chain that blocks access to the active site is chemically removed as the substrate approaches.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following best describes a typical mechanism for an enzyme-catalyzed reaction?
A) Slow formation of an enzyme-substrate complex, then fast formation of products and regeneration of enzyme
B) Fast formation of an enzyme-substrate complex, then slow formation of products and regeneration of enzyme
C) Slow binding of a cofactor to the substrate, then fast reaction with the enzyme to form an enzyme-substrate complex
D) Fast binding of a cofactor to the substrate, then slow reaction with the enzyme to form an enzyme-substrate complex
E) Slow formation of regenerated enzyme, then fast formation of products
A) Slow formation of an enzyme-substrate complex, then fast formation of products and regeneration of enzyme
B) Fast formation of an enzyme-substrate complex, then slow formation of products and regeneration of enzyme
C) Slow binding of a cofactor to the substrate, then fast reaction with the enzyme to form an enzyme-substrate complex
D) Fast binding of a cofactor to the substrate, then slow reaction with the enzyme to form an enzyme-substrate complex
E) Slow formation of regenerated enzyme, then fast formation of products

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
44
The function of an automobile catalytic converter is to convert
A) CO to CO2 and NO to N2.
B) C to CO and NO to N2.
C) CO to CO2 and O2 to O3.
D) CO to CO2 and NO to N2O.
E) CO to CO2 and N2 to NO.
A) CO to CO2 and NO to N2.
B) C to CO and NO to N2.
C) CO to CO2 and O2 to O3.
D) CO to CO2 and NO to N2O.
E) CO to CO2 and N2 to NO.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
45
In a heterogeneous chemical reaction requiring the use of a solid catalyst, the rate of the reaction could not be increased by increasing the concentration of reactants, but was doubled by doubling the amount of solid catalyst added. This probably indicated that
A) a minimum amount of catalyst was required to make the catalyst active
B) the active sites on the initial amount of catalyst were fully saturated
C) catalysts should be considered as just another chemical reactant
D) the catalyst actually does not have any effect on the rate of this reaction at all
E) all of these
A) a minimum amount of catalyst was required to make the catalyst active
B) the active sites on the initial amount of catalyst were fully saturated
C) catalysts should be considered as just another chemical reactant
D) the catalyst actually does not have any effect on the rate of this reaction at all
E) all of these

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following factors increases the rate of a chemical reaction by increasing the fraction of molecules with energy in excess of a given value?
A) increasing the concentration of products
B) increasing the concentration of reactants
C) increasing the temperature
D) adding a catalyst
E) increasing the surface area of a phase interface
A) increasing the concentration of products
B) increasing the concentration of reactants
C) increasing the temperature
D) adding a catalyst
E) increasing the surface area of a phase interface

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
47
The substrate in an enzyme-controlled biological reaction is a
A) molecule serving as a rigid support for the enzyme.
B) molecule that is acted on by the catalyst.
C) species necessary for the proper functioning of the enzyme.
D) biological molecule used for storage of energy.
E) catalyst for that chemical reaction.
A) molecule serving as a rigid support for the enzyme.
B) molecule that is acted on by the catalyst.
C) species necessary for the proper functioning of the enzyme.
D) biological molecule used for storage of energy.
E) catalyst for that chemical reaction.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
48
The solid catalysts used in many industrial processes where the reactants are liquids or gases are referred to as
A) silicon catalysts.
B) zeolites.
C) homogeneous catalysts.
D) heterogeneous catalysts.
E) enzymes.
A) silicon catalysts.
B) zeolites.
C) homogeneous catalysts.
D) heterogeneous catalysts.
E) enzymes.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
49
An enzyme is a(n)
A) organic or inorganic molecule or ion necessary for proper functioning of biological catalysts.
B) catalyst for chemical reactions in a living system.
C) protein molecule whose primary function is transport of insoluble molecules in the bloodstream.
D) molecule that is acted on by a catalyst in a living system.
E) biological molecule used for storage of energy.
A) organic or inorganic molecule or ion necessary for proper functioning of biological catalysts.
B) catalyst for chemical reactions in a living system.
C) protein molecule whose primary function is transport of insoluble molecules in the bloodstream.
D) molecule that is acted on by a catalyst in a living system.
E) biological molecule used for storage of energy.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
50
Which statement is true about a catalyst?
A) It increases the activation energy involved in a reaction.
B) It can be the same phase as the reactants (heterogeneous) or a different phase (homogeneous).
C) It is formed during an early step in the reaction and consumed in a later step.
D) It does not participate in the reaction.
E) It does not appear in the overall equation for the reaction.
A) It increases the activation energy involved in a reaction.
B) It can be the same phase as the reactants (heterogeneous) or a different phase (homogeneous).
C) It is formed during an early step in the reaction and consumed in a later step.
D) It does not participate in the reaction.
E) It does not appear in the overall equation for the reaction.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
51
Exhibit 12-2 For the following question(s), consider the following reaction mechanism:
 Refer to Exhibit 12-2. The intermediates in this reaction are
Refer to Exhibit 12-2. The intermediates in this reaction are
A) Z and V.
B) X and Y.
C) Y only.
D) W and Y.
E) There are no intermediates in this reaction.
 Refer to Exhibit 12-2. The intermediates in this reaction are
Refer to Exhibit 12-2. The intermediates in this reaction areA) Z and V.
B) X and Y.
C) Y only.
D) W and Y.
E) There are no intermediates in this reaction.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
52
If the rate law for an elementary reaction is second order, we can conclude that
A) no catalysts are involved in the reaction.
B) no intermediates are formed in the reaction.
C) additional experiments at different temperatures are needed to determine the molecularity of the reaction.
D) the elementary reaction involves a collision between two molecules.
E) the elementary reaction is unimolecular.
A) no catalysts are involved in the reaction.
B) no intermediates are formed in the reaction.
C) additional experiments at different temperatures are needed to determine the molecularity of the reaction.
D) the elementary reaction involves a collision between two molecules.
E) the elementary reaction is unimolecular.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
53
The kinetics of a reaction are observed to be third-order. The least likely mechanism
A) involves three molecules reacting together in a single step.
B) involves two molecules reacting in one step, and the third in a subsequent step.
C) involves a single intermediate.
D) involves more than one intermediate.
E) involves a fast step followed by a slow step.
A) involves three molecules reacting together in a single step.
B) involves two molecules reacting in one step, and the third in a subsequent step.
C) involves a single intermediate.
D) involves more than one intermediate.
E) involves a fast step followed by a slow step.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
54
A reaction intermediate is a species which
A) speeds up a reaction by lowering the activation energy.
B) slows down a reaction by raising the activation energy.
C) is always produced in the rate-determining step.
D) is always produced in an early step and consumed in a later step.
E) is always consumed in an early step and regenerated in a later step.
A) speeds up a reaction by lowering the activation energy.
B) slows down a reaction by raising the activation energy.
C) is always produced in the rate-determining step.
D) is always produced in an early step and consumed in a later step.
E) is always consumed in an early step and regenerated in a later step.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
55
The rate constant for the reaction 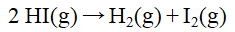 1 doubles on raising the temperature from 410 C to 425 C. The activation energy, in J/mol, is
1 doubles on raising the temperature from 410 C to 425 C. The activation energy, in J/mol, is
A) 5.3 × 105.
B) 1.8 × 105.
C) 9.1 × 104.
D) 6.7 × 104.
E) -1.9 × 105.
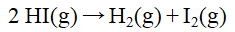 1 doubles on raising the temperature from 410 C to 425 C. The activation energy, in J/mol, is
1 doubles on raising the temperature from 410 C to 425 C. The activation energy, in J/mol, isA) 5.3 × 105.
B) 1.8 × 105.
C) 9.1 × 104.
D) 6.7 × 104.
E) -1.9 × 105.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
56
The ____of a multistep reaction can be predicted unambiguously from the ____, but not vice versa.
A) mechanism; kinetic rate law
B) mechanism; Arrhenius Equation
C) activation energy; mechanism
D) kinetic rate law; mechanism
E) mechanism; activation energy
A) mechanism; kinetic rate law
B) mechanism; Arrhenius Equation
C) activation energy; mechanism
D) kinetic rate law; mechanism
E) mechanism; activation energy

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
57
If an elementary reaction involves the collision of two identical molecules, then the elementary reaction is ____, and the kinetics will be ____.
A) unimolecular; zeroth-order.
B) unimolecular; first-order.
C) unimolecular; second-order.
D) bimolecular; first-order.
E) bimolecular; second-order.
A) unimolecular; zeroth-order.
B) unimolecular; first-order.
C) unimolecular; second-order.
D) bimolecular; first-order.
E) bimolecular; second-order.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following factors increases the rate of a chemical reaction by decreasing the activation energy barrier?
A) increasing the temperature
B) decreasing the temperature
C) adding a catalyst
D) increasing the surface area of a phase interface
E) increasing the concentration of reactants
A) increasing the temperature
B) decreasing the temperature
C) adding a catalyst
D) increasing the surface area of a phase interface
E) increasing the concentration of reactants

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
59
Which statement, comparing catalyzed and uncatalyzed versions of a reaction, is incorrect?
A) The initial and final energy states are the same.
B) The reaction mechanisms are different.
C) The reaction progress diagrams are different.
D) The balanced equation for the overall reaction is the same.
E) The activation energy is lower in the uncatalyzed reaction.
A) The initial and final energy states are the same.
B) The reaction mechanisms are different.
C) The reaction progress diagrams are different.
D) The balanced equation for the overall reaction is the same.
E) The activation energy is lower in the uncatalyzed reaction.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
60
Exhibit 12-2 For the following question(s), consider the following reaction mechanism:
 Refer to Exhibit 12-2. The catalysts in this reaction are
Refer to Exhibit 12-2. The catalysts in this reaction are
A) Z and V.
B) X and Y.
C) Y only.
D) W and Y.
E) There are no catalysts in this reaction.
 Refer to Exhibit 12-2. The catalysts in this reaction are
Refer to Exhibit 12-2. The catalysts in this reaction areA) Z and V.
B) X and Y.
C) Y only.
D) W and Y.
E) There are no catalysts in this reaction.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
61
The table below gives the rate constant for a certain reaction at
a. the activation energy for this reaction.
b. the frequency factor.
c. the value of k at 1100K.

a. the activation energy for this reaction.
b. the frequency factor.
c. the value of k at 1100K.


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
62
The table below gives the concentration of a substance A as a function of time at 473 K.

a. Establish whether the reaction is zeroth, first or second-order in A.
b. Calculate the rate constant at 473 K for this reaction.

a. Establish whether the reaction is zeroth, first or second-order in A.
b. Calculate the rate constant at 473 K for this reaction.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
63
Phosgene, COCl2, is formed from Cl2 and CO at high temperatures. The mechanism is thought to be:

What is the rate law predicted for this mechanism?

What is the rate law predicted for this mechanism?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
64
Consider this proposed mechanism for the reaction of NO2 and CO:
 a. Write the overall reaction represented by this mechanism.
a. Write the overall reaction represented by this mechanism.
b. What are the intermediates in this reaction, if any?
c. What are the catalysts in this reaction, if any?
d. Write the rate law predicted by the mechanism above.
e. Write the rate law you would predict if the reaction went in a single step.
 a. Write the overall reaction represented by this mechanism.
a. Write the overall reaction represented by this mechanism.b. What are the intermediates in this reaction, if any?
c. What are the catalysts in this reaction, if any?
d. Write the rate law predicted by the mechanism above.
e. Write the rate law you would predict if the reaction went in a single step.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
65
The conversion of substance A to substance B has DE = -35 kJ mol-1 and an activation energy, Ea, of 25 kJ mol-1. Sketch a reaction diagram, and on it clearly indicate A, B, the activated complex (C), DE and Ea.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
66
When studying kinetics, it is more accurate to measure instantaneous reaction rates at particular times rather than the average rates of reaction over time intervals. Explain why.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck


