Exam 12: Chemical Kinetics: Rates of Reactions
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
The decomposition of N2O(g) to nitrogen and oxygen has the rate law 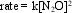 where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
D
Which statement, comparing catalyzed and uncatalyzed versions of a reaction, is incorrect?
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
E
Consider this proposed mechanism for the reaction of NO2 and CO:
 a. Write the overall reaction represented by this mechanism.
b. What are the intermediates in this reaction, if any?
c. What are the catalysts in this reaction, if any?
d. Write the rate law predicted by the mechanism above.
e. Write the rate law you would predict if the reaction went in a single step.
a. Write the overall reaction represented by this mechanism.
b. What are the intermediates in this reaction, if any?
c. What are the catalysts in this reaction, if any?
d. Write the rate law predicted by the mechanism above.
e. Write the rate law you would predict if the reaction went in a single step.
Free
(Essay)
4.8/5  (30)
(30)
Correct Answer:
a. NO2 + CO ® NO + CO2
b. NO3 is an intermediate; it is generated in the first step and used up in the second step.
c. One of the NO2 molecules is a catalyst, since it is used up in the first step and regenerated in the second step.
d. Since the first step is slow (i.e., rate-determining), the rate law is
rate = k[NO2]2
e. Rate = k[NO2][CO].
A certain reaction is studied at room temperature, and gives a straight line plot for 1/[reactant] versus time. Which one of the following statements is true?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following factors will affect the rate of a homogeneous reaction?
(Multiple Choice)
4.8/5  (36)
(36)
The activation energy in the Arrhenius equation can best be described as
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following factors will affect the rate of a reaction only if it is heterogeneous?
(Multiple Choice)
4.8/5  (39)
(39)
The rate law for a given reaction is rate = k[reactant]2, with k = 2.64 × 10-4 M-1 min-1. If the initial concentration is 0.0250 M, what is the initial rate, with the correct units?
(Multiple Choice)
4.9/5  (33)
(33)
If the rate law for an elementary reaction is second order, we can conclude that
(Multiple Choice)
5.0/5  (34)
(34)
For a reaction that is zeroth-order with respect to reactant A, and nth-order overall
(Multiple Choice)
4.8/5  (24)
(24)
The kinetics of a reaction are observed to be third-order. The least likely mechanism
(Multiple Choice)
4.8/5  (25)
(25)
The frequency factor in the Arrhenius equation can best be described as
(Multiple Choice)
4.8/5  (26)
(26)
If a reaction is first-order with respect to each of its two reactants, then the rate of the reaction is
(Multiple Choice)
4.9/5  (34)
(34)
Phosgene, COCl2, is formed from Cl2 and CO at high temperatures. The mechanism is thought to be:
 What is the rate law predicted for this mechanism?
What is the rate law predicted for this mechanism?
(Essay)
4.9/5  (43)
(43)
For a zero-, first- or second-order reaction  the order of reaction with respect to reactant A may be determined
the order of reaction with respect to reactant A may be determined
(Multiple Choice)
4.9/5  (39)
(39)
Exhibit 12-1 The following question(s) relate to the reaction between water and a complex ion of Co2+; the rate varies with concentration as follows. (The rate has no dependence on [H2O].)
![Exhibit 12-1 The following question(s) relate to the reaction between water and a complex ion of Co<sup>2+</sup>; the rate varies with concentration as follows. (The rate has no dependence on [H<sub>2</sub>O].) Refer to Exhibit 12-1. The value and units of the rate constant are:](https://storage.examlex.com/TB5061/11ea782d_fd82_2e16_bbd5_37d006af06ca_TB5061_00.jpg) Refer to Exhibit 12-1. The value and units of the rate constant are:
Refer to Exhibit 12-1. The value and units of the rate constant are:
(Multiple Choice)
4.8/5  (29)
(29)
Showing 1 - 20 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)