Deck 10: Gases and the Atmosphere
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/57
Play
Full screen (f)
Deck 10: Gases and the Atmosphere
1
Which description of the kinetic-molecular theory of gases is true?
A) The space between two gas molecules is much greater than the size of each gas molecule.
B) The forces of attraction and repulsion do not exist for gas molecules.
C) Colliding gas molecules have the same speed before and after the collision.
D) Gas molecules move randomly in all directions with the same speed.
E) Gas molecules lose energy with every collision and will eventually stop moving.
A) The space between two gas molecules is much greater than the size of each gas molecule.
B) The forces of attraction and repulsion do not exist for gas molecules.
C) Colliding gas molecules have the same speed before and after the collision.
D) Gas molecules move randomly in all directions with the same speed.
E) Gas molecules lose energy with every collision and will eventually stop moving.
The space between two gas molecules is much greater than the size of each gas molecule.
2
Which correctly describes the relationship between the applied pressure on a gas and its volume at constant temperature?
A) as one increases the other increases
B) they are unrelated
C) they are directly proportional
D) they are irreversibly proportional
E) they are inversely proportional
A) as one increases the other increases
B) they are unrelated
C) they are directly proportional
D) they are irreversibly proportional
E) they are inversely proportional
they are inversely proportional
3
Convert 699 torr to
1) atm
2) mmHg
3) kPa.

1) atm
2) mmHg
3) kPa.

D
4
What happens to a helium balloon at a party on a hot day if the balloon was filled on a cold morning?
A) The balloon shrinks because the warmer day temperature speeds up the helium atoms and increases the number of atoms that escape through the inner wall of the balloon.
B) The balloon expands because the warmer day temperature causes the helium atoms to be less attracted to each other and allows them to occupy more space inside the balloon.
C) The balloon expands because the warmer day temperature speeds up the helium atoms and increases the force and frequency of gas collisions on the inner wall of the balloon.
D) The balloon shrinks because the warmer day temperature causes the helium atoms to be more attracted to each other and allows them to occupy less space inside the balloon.
E) The balloon keeps the same volume because the amount of gas inside the balloon does not change.
A) The balloon shrinks because the warmer day temperature speeds up the helium atoms and increases the number of atoms that escape through the inner wall of the balloon.
B) The balloon expands because the warmer day temperature causes the helium atoms to be less attracted to each other and allows them to occupy more space inside the balloon.
C) The balloon expands because the warmer day temperature speeds up the helium atoms and increases the force and frequency of gas collisions on the inner wall of the balloon.
D) The balloon shrinks because the warmer day temperature causes the helium atoms to be more attracted to each other and allows them to occupy less space inside the balloon.
E) The balloon keeps the same volume because the amount of gas inside the balloon does not change.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
5
Which statement is false?
A) Atmospheric gases are an example of gases mixing completely.
B) An exhaled breath of air will disperse throughout the entire atmosphere.
C) A gas inside a container will remain inside if the container is opened.
D) Atmospheric pressure is a result of gas molecules exerting pressure on their surroundings.
E) An air pump demonstrates that gases can be compressed.
A) Atmospheric gases are an example of gases mixing completely.
B) An exhaled breath of air will disperse throughout the entire atmosphere.
C) A gas inside a container will remain inside if the container is opened.
D) Atmospheric pressure is a result of gas molecules exerting pressure on their surroundings.
E) An air pump demonstrates that gases can be compressed.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
6
What is the correct name for the atmospheric layer in which weather phenomena occur?
A) ionosphere
B) thermosphere
C) mesosphere
D) stratosphere
E) troposphere
A) ionosphere
B) thermosphere
C) mesosphere
D) stratosphere
E) troposphere

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
7
If, at constant pressure, the absolute temperature of a flask equipped with a moveable piston is reduced to half its original absolute temperature, what must be the new volume?
A) 1/4 the original volume
B) 1/2 the original volume
C) two times the original volume
D) four times the original volume
E) equal to the original volume
A) 1/4 the original volume
B) 1/2 the original volume
C) two times the original volume
D) four times the original volume
E) equal to the original volume

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
8
Which concept is not associated with the kinetic-molecular theory of gases?
A) No energy is lost when gas molecules collide with each other.
B) Gas molecules move randomly in all possible directions at various speeds.
C) The average kinetic energy of gas molecules increases as the temperature increases.
D) Different gases with the same temperature, pressure, and volume behave much differently.
E) The forces of attraction and repulsion between separate gas molecules are minimal.
A) No energy is lost when gas molecules collide with each other.
B) Gas molecules move randomly in all possible directions at various speeds.
C) The average kinetic energy of gas molecules increases as the temperature increases.
D) Different gases with the same temperature, pressure, and volume behave much differently.
E) The forces of attraction and repulsion between separate gas molecules are minimal.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
9
Clean air
A) is pure oxygen.
B) contains mostly oxygen, some nitrogen and nothing else.
C) contains mostly nitrogen, some oxygen and nothing else.
D) contains mostly nitrogen, some oxygen, and a very small amount of several other gases.
E) has all of its nitrogen removed.
A) is pure oxygen.
B) contains mostly oxygen, some nitrogen and nothing else.
C) contains mostly nitrogen, some oxygen and nothing else.
D) contains mostly nitrogen, some oxygen, and a very small amount of several other gases.
E) has all of its nitrogen removed.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
10
Arrange the following gaseous substances in order of decreasing average molecular speed at 25°C. 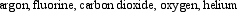
A) helium > oxygen > fluorine > carbon dioxide > argon
B) helium > oxygen > fluorine > argon > carbon dioxide
C) argon > oxygen > helium > carbon dioxide > fluorine
D) carbon dioxide > argon > fluorine > oxygen > helium
E) carbon dioxide > fluorine > argon > oxygen > helium
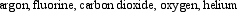
A) helium > oxygen > fluorine > carbon dioxide > argon
B) helium > oxygen > fluorine > argon > carbon dioxide
C) argon > oxygen > helium > carbon dioxide > fluorine
D) carbon dioxide > argon > fluorine > oxygen > helium
E) carbon dioxide > fluorine > argon > oxygen > helium

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
11
Based on temperature, there are four main layers to our atmosphere. What is the correct name for the layer in which we live?
A) troposphere
B) thermosphere
C) stratosphere
D) mesosphere
E) ionosphere
A) troposphere
B) thermosphere
C) stratosphere
D) mesosphere
E) ionosphere

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
12
If a 2.0-liter sample of gas experiences a decrease in pressure from 1.74 atm to 0.555 atm at 25°C, what is the resulting volume (in L) at 25°C?
A) 0.48 L
B) 0.64 L
C) 1.9 L
D) 6.3 L
E) 20 L
A) 0.48 L
B) 0.64 L
C) 1.9 L
D) 6.3 L
E) 20 L

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
13
What is the correct name for the atmospheric layer in which commercial jets fly?
A) ionosphere
B) thermosphere
C) mesosphere
D) stratosphere
E) troposphere
A) ionosphere
B) thermosphere
C) mesosphere
D) stratosphere
E) troposphere

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
14
If, at constant temperature, the volume of a flask equipped with a moveable piston is reduced to 1/4 its original volume, what must happen to the pressure?
A) It is increased by a factor of four.
B) It is increased by a factor of eight.
C) It is reduced to 1/4 the original pressure.
D) It is reduced to 1/8 the original pressure.
E) It remains the same as the original pressure.
A) It is increased by a factor of four.
B) It is increased by a factor of eight.
C) It is reduced to 1/4 the original pressure.
D) It is reduced to 1/8 the original pressure.
E) It remains the same as the original pressure.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
15
Which statement summarizes Charles's Law?
A) The volume of a fixed amount of gas at a constant temperature is inversely proportional to its pressure.
B) The pressure of a variable amount of gas at different pressures is directly proportional to its absolute temperature.
C) The volume of a fixed amount of gas at a constant pressure is directly proportional to its absolute temperature.
D) The volume of a fixed amount of gas at a constant pressure is inversely proportional to its absolute temperature.
E) The pressure of a fixed amount of gas at variable pressure is directly proportional to its absolute temperature.
A) The volume of a fixed amount of gas at a constant temperature is inversely proportional to its pressure.
B) The pressure of a variable amount of gas at different pressures is directly proportional to its absolute temperature.
C) The volume of a fixed amount of gas at a constant pressure is directly proportional to its absolute temperature.
D) The volume of a fixed amount of gas at a constant pressure is inversely proportional to its absolute temperature.
E) The pressure of a fixed amount of gas at variable pressure is directly proportional to its absolute temperature.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
16
When the external pressure on a balloon is decreased at constant temperature, what happens to the gas inside?
A) The collisions become more elastic.
B) The gas undergoes no changes.
C) The volume increases.
D) The molecules increase in rate of movement.
E) The volume decreases.
A) The collisions become more elastic.
B) The gas undergoes no changes.
C) The volume increases.
D) The molecules increase in rate of movement.
E) The volume decreases.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
17
Which is a mathematical representation of Charles's Law?
A) P/T = constant
B) PV = constant
C) V × T = constant
D) n × T = constant
E) V/T = constant
A) P/T = constant
B) PV = constant
C) V × T = constant
D) n × T = constant
E) V/T = constant

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
18
Which is the most abundant gas found in dry air at sea level?
A) H2
B) Cl2
C) N2
D) O2
E) Ne
A) H2
B) Cl2
C) N2
D) O2
E) Ne

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
19
At 20°C, a sample of gas has a volume of 2.51 L at 795 torr. What is the new pressure (in atm) if the volume is increased to 6.46 L at the same temperature?
A) 2.69 atm
B) 0.813 atm
C) 0.406 atm
D) 309 atm
E) 2050 atm
A) 2.69 atm
B) 0.813 atm
C) 0.406 atm
D) 309 atm
E) 2050 atm

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
20
Which statement about ideal gases and the gas laws is false?
A) Most gases are ideal or almost ideal gases at room temperature and atmospheric pressure.
B) The laws are summarized as equations that describe the behavior of ideal gases.
C) Variables that appear in the laws are temperature, pressure, volume, and amount of gas.
D) A gas will behave ideally under all conditions.
E) The laws describe the macroscopic behavior of ideal gases.
A) Most gases are ideal or almost ideal gases at room temperature and atmospheric pressure.
B) The laws are summarized as equations that describe the behavior of ideal gases.
C) Variables that appear in the laws are temperature, pressure, volume, and amount of gas.
D) A gas will behave ideally under all conditions.
E) The laws describe the macroscopic behavior of ideal gases.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
21
A sample of chlorine gas (Cl2) occupies 1.65 L at 1.0 atm and 308 K. What pressure (in mm Hg) will the sample exert at 3.00 L and 51.0 °C?
A) 0.579 mm Hg
B) 69.2 mm Hg
C) 397 mm Hg
D) 440 mm Hg
E) 579 mm Hg
A) 0.579 mm Hg
B) 69.2 mm Hg
C) 397 mm Hg
D) 440 mm Hg
E) 579 mm Hg

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
22
Determine the volume (in L) of Cl2(g) required to carry out the following reaction at 794 torr and 625°C using 15.0 g of Fe. The value of R = 0.0821 L atm mol-1 K-1. 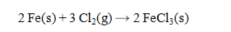
A) 1.06 × 103 L
B) 0.0374 L
C) 19.0 L
D) 19.8 L
E) 28.4 L
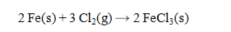
A) 1.06 × 103 L
B) 0.0374 L
C) 19.0 L
D) 19.8 L
E) 28.4 L

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
23
A balloon filled with gas is heated from 30.0°C to 197.0°C at constant pressure. If the original volume of the balloon was 1.560 L, what is the volume (in L) of the gas after heating?
A) 0.238 L
B) 1.02 L
C) 1.01 L
D) 2.42 L
E) 10.2 L
A) 0.238 L
B) 1.02 L
C) 1.01 L
D) 2.42 L
E) 10.2 L

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
24
Aluminum and oxygen react to form aluminum oxide. How many L of oxygen at 296 K and 1.4 atm of pressure are necessary to form 3.25 g of aluminum oxide? R = 0.0821 L atm mol-1 K-1.
A) 6.5 × 10-2 L
B) 0.55 L
C) 0.83 L
D) 1.3 L
E) 56 L
A) 6.5 × 10-2 L
B) 0.55 L
C) 0.83 L
D) 1.3 L
E) 56 L

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
25
What is the pressure (in atm) exerted by a 2.00 mole sample of gas that occupies 2.50 L at 307.15 K? The value of R = 0.0821 L atm mol-1 K-1.
A) 40.3 atm
B) 20.2 atm
C) 80.7 atm
D) 0.0496 atm
E) 38.1 atm
A) 40.3 atm
B) 20.2 atm
C) 80.7 atm
D) 0.0496 atm
E) 38.1 atm

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
26
Calcium carbonate decomposes upon heating to form calcium oxide and carbon dioxide. A 1.75-g sample of calcium carbonate is placed in an empty, sealed 2.00-L steel container. The container is heated to 625°C and then allowed to cool to 25°C. What is the final pressure (in atm) inside the container? The value of R = 0.0821 L atm mol-1 K-1.
A) 0.21 atm
B) 0.43 atm
C) 0.64 atm
D) 1.8 atm
E) 59 atm
A) 0.21 atm
B) 0.43 atm
C) 0.64 atm
D) 1.8 atm
E) 59 atm

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
27
The following is the reaction that occurs in automobile airbags: 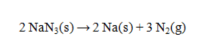 How many grams of sodium azide (NaN3) are required to produce 19.00 L of N2 at 293K and 775 mm Hg? R = 0.0821 L atm mol-1 K-1.
How many grams of sodium azide (NaN3) are required to produce 19.00 L of N2 at 293K and 775 mm Hg? R = 0.0821 L atm mol-1 K-1.
A) 34.9 g
B) 52.0 g
C) 2.66 × 104 g
D) 3.89 × 105 g
E) 5.80 × 105 g
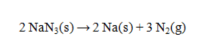 How many grams of sodium azide (NaN3) are required to produce 19.00 L of N2 at 293K and 775 mm Hg? R = 0.0821 L atm mol-1 K-1.
How many grams of sodium azide (NaN3) are required to produce 19.00 L of N2 at 293K and 775 mm Hg? R = 0.0821 L atm mol-1 K-1.A) 34.9 g
B) 52.0 g
C) 2.66 × 104 g
D) 3.89 × 105 g
E) 5.80 × 105 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
28
What conditions are referred to as standard temperature and pressure (STP) for gas behavior?
A) 0°C and 1 atm
B) 0°C and 1 mm Hg
C) 273°C and 1 atm
D) 25°C and 1 atm
E) 298 K and 760 torr
A) 0°C and 1 atm
B) 0°C and 1 mm Hg
C) 273°C and 1 atm
D) 25°C and 1 atm
E) 298 K and 760 torr

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
29
How are the pressure and the absolute temperature of a gas related for a given number of moles at constant volume?
A) They are exactly opposites.
B) They are not related.
C) They are directly proportional.
D) They are indirectly proportional.
E) Not enough information is given to determine the relationship.
A) They are exactly opposites.
B) They are not related.
C) They are directly proportional.
D) They are indirectly proportional.
E) Not enough information is given to determine the relationship.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
30
A 125.0-mL contains a gas at 27°C and 760 mm Hg of pressure. The gas is pumped into an empty 1.50-L flask also at a temperature of 27°C. What is the pressure (in atm) of the gas in the new flask?
A) 0.0833 atm
B) 63.3 atm
C) 83.3 atm
D) 63300 atm
E) 12.0 atm
A) 0.0833 atm
B) 63.3 atm
C) 83.3 atm
D) 63300 atm
E) 12.0 atm

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
31
What volume (in L) of oxygen at 298K and 1.50 atm is required for the complete combustion of 25.0 g of hexane? The value of R = 0.0821 L atm mol-1 K-1. 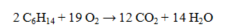
A) 4.73 L
B) 0.397 L
C) 34.2 L
D) 45.0 L
E) 407 L
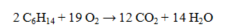
A) 4.73 L
B) 0.397 L
C) 34.2 L
D) 45.0 L
E) 407 L

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
32
Suppose that at STP a gas occupies 10.2 L. At what temperature (°C) will the same gas occupy 15000 mL at 1550 torr?
A) -273 °C
B) 1.22 × 10-3 °C
C) 545 °C
D) 6.22 × 105 °C
E) undefined due to division by zero
A) -273 °C
B) 1.22 × 10-3 °C
C) 545 °C
D) 6.22 × 105 °C
E) undefined due to division by zero

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
33
What is the density of oxygen gas (O2) at STP? R = 0.0821 L atm mol-1 K-1.
A) 1.43 g/L
B) 0.714 g/L
C) 1.31 g/L
D) 1090 g/L
E) 716 g/L
A) 1.43 g/L
B) 0.714 g/L
C) 1.31 g/L
D) 1090 g/L
E) 716 g/L

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
34
Derive the gas constant (in L torr mol-1 K-1) given that 1.000 mole of a gas occupying 22.4 L at 0.00°C and 1.00 atm.
A) 1.22 × 10-3 L torr mol-1 K-1
B) 8.21 × 10-2 L torr mol-1 K-1
C) 1.60 × 10-2 L torr mol-1 K-1
D) 62.3 L torr mol-1 K-1
E) undefined due to division by zero
A) 1.22 × 10-3 L torr mol-1 K-1
B) 8.21 × 10-2 L torr mol-1 K-1
C) 1.60 × 10-2 L torr mol-1 K-1
D) 62.3 L torr mol-1 K-1
E) undefined due to division by zero

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
35
What is the molar mass of an unknown gas if 3.7 g of the gas occupies 450 mL at 20.0°C and 2.0 atm? The value of R = 0.0821 L atm mol-1 K-1.
A) 3.7 × 10-2 g/mol
B) 9.9 × 10-3 g/mol
C) 6.8 g/mol
D) 98.9 g/mol
E) 5.5 × 103 g/mol
A) 3.7 × 10-2 g/mol
B) 9.9 × 10-3 g/mol
C) 6.8 g/mol
D) 98.9 g/mol
E) 5.5 × 103 g/mol

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
36
Which is a summary of Avogadro's Law?
A) At constant temperature and pressure, the volume of a gas is equal to the number of moles of the gas.
B) At constant temperature and pressure, the volume of a gas is inversely proportional to the number of moles of the gas.
C) At variable temperature and pressure, the volume of a gas is directly proportional to the number of moles of the gas.
D) At constant temperature and pressure, the volume of a gas is not related to the number of moles of the gas.
E) At constant temperature and pressure, the volume of a gas is directly proportional to the number of moles of the gas.
A) At constant temperature and pressure, the volume of a gas is equal to the number of moles of the gas.
B) At constant temperature and pressure, the volume of a gas is inversely proportional to the number of moles of the gas.
C) At variable temperature and pressure, the volume of a gas is directly proportional to the number of moles of the gas.
D) At constant temperature and pressure, the volume of a gas is not related to the number of moles of the gas.
E) At constant temperature and pressure, the volume of a gas is directly proportional to the number of moles of the gas.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
37
If at 29°C and 2.50 atm of pressure, a gas occupies 5.99 L, what volume (in L) will the same gas occupy at 19°C and 1.20 atm of pressure?
A) 2.04 × 10-4 L
B) 6.04 × 10-1 L
C) 8.18 L
D) 12.1 L
E) 20.0 L
A) 2.04 × 10-4 L
B) 6.04 × 10-1 L
C) 8.18 L
D) 12.1 L
E) 20.0 L

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the number of moles of a gas that occupy 7.3 × 103 mL at STP. The value of R = 0.0821 L atm mol-1 K-1.
A) 0.33 mol
B) 3.1 mol
C) 3.3 × 102 mol
D) 2.4 × 104 mol
E) undefined due to division by zero
A) 0.33 mol
B) 3.1 mol
C) 3.3 × 102 mol
D) 2.4 × 104 mol
E) undefined due to division by zero

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
39
Suppose that in a known volume of He there are 6.022 × 1023 molecules (4.00 g) present at 1.00 atm and 0°C. How many molecules and how many grams of Cl2 are there under the same conditions?
A) 6.022 × 1023 molecules and 35.45 g
B) 1.00 molecules and 4.00 g
C) 6.022 × 1023 molecules and 70.90 g
D) 2(6.022 × 1023) molecules and 35.45 g
E) 8(6.022 × 1023) molecules and 70.90 g
A) 6.022 × 1023 molecules and 35.45 g
B) 1.00 molecules and 4.00 g
C) 6.022 × 1023 molecules and 70.90 g
D) 2(6.022 × 1023) molecules and 35.45 g
E) 8(6.022 × 1023) molecules and 70.90 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
40
Which is a mathematical representation of Avogadro's Law?
A) V = constant × n
B) n = constant
C) 1/T = constant
D) n/V = constant
E) P/V = constant
A) V = constant × n
B) n = constant
C) 1/T = constant
D) n/V = constant
E) P/V = constant

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
41
Which statement about greenhouse gases and global warming is false?
A) Global warming increases with increased carbon dioxide in the atmosphere.
B) Planting trees would reduce atmospheric carbon dioxide.
C) Global warming can cause flooding, weather changes, and changes in agricultural patterns.
D) Human activity is the sole source of atmospheric carbon dioxide.
E) Computer models are used to predict future global temperature changes.
A) Global warming increases with increased carbon dioxide in the atmosphere.
B) Planting trees would reduce atmospheric carbon dioxide.
C) Global warming can cause flooding, weather changes, and changes in agricultural patterns.
D) Human activity is the sole source of atmospheric carbon dioxide.
E) Computer models are used to predict future global temperature changes.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
42
Under what conditions do real gases most closely approximate the behavior of ideal gases?
A) high pressure and high temperature
B) high pressure and low temperature
C) standard temperature and pressure
D) low pressure and high temperature
E) low pressure and low temperature
A) high pressure and high temperature
B) high pressure and low temperature
C) standard temperature and pressure
D) low pressure and high temperature
E) low pressure and low temperature

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
43
A 5.00 L flask contains 3.50 g of sulfur trioxide, 2.45 g of carbon monoxide, and 3.99 g of argon all at 35°C. What is the pressure (in atm) in the flask?
A) 0.221 atm
B) 0.234 atm
C) 50.5 atm
D) 5.70 atm
E) 1.17 atm
A) 0.221 atm
B) 0.234 atm
C) 50.5 atm
D) 5.70 atm
E) 1.17 atm

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
44
Which two assumptions of the Kinetic-Molecular Theory need to be altered to account for the behavior of real gases?
I. gas molecules have no volume
II. gas molecules move in random motion
III. gas molecules undergo no attractive or repulsive force toward one another
IV. gas molecules undergo perfectly elastic collision
A) I and II
B) I and III
C) II and III
D) II and IV
E) III and IV
I. gas molecules have no volume
II. gas molecules move in random motion
III. gas molecules undergo no attractive or repulsive force toward one another
IV. gas molecules undergo perfectly elastic collision
A) I and II
B) I and III
C) II and III
D) II and IV
E) III and IV

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
45
A mixture of H2(g) and Cl2(g) exerts a total pressure of 2.50 atm. If the mole fraction of H2 is 0.490, what is the partial pressure (in atm) of H2?
A) 0.253 atm
B) 0.396 atm
C) 0.606 atm
D) 1.23 atm
E) 2.50 atm
A) 0.253 atm
B) 0.396 atm
C) 0.606 atm
D) 1.23 atm
E) 2.50 atm

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
46
A mixture of He, Ne and Ar has a total pressure of 0.80 atm and is found to contain 0.55 mol He, 0.94 mol Ne and 0.35 mol Ar. What is the partial pressure (in atm) of each gas? He Ne Ar
A) 0.095 atm 0.16 atm 0.060 atm
B) 0.44 atm 0.75 atm 0.28 atm
C) 0.24 atm 0.41 atm 0.15 atm
D) 2.7 atm 1.6 atm 4.2 atm
E) 1.5 atm .85 atm 1.5 atm
A) 0.095 atm 0.16 atm 0.060 atm
B) 0.44 atm 0.75 atm 0.28 atm
C) 0.24 atm 0.41 atm 0.15 atm
D) 2.7 atm 1.6 atm 4.2 atm
E) 1.5 atm .85 atm 1.5 atm

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
47
Hydrochloric acid reacts with magnesium to produce hydrogen gas and magnesium chloride. Reacting magnesium with excess hydrochloric acid produces 1204 mL of hydrogen gas that is collected over water. How many grams of hydrogen gas are produced at 25.0 °C and 1.00 atm? The vapor pressure of water at 25.0 °C is 23.8 mm Hg.
A) 9.61 × 10-2 g
B) 5.87 × 10-1 g
C) 1.18 g
D) 4.77 × 10-2 g
E) 7.24 × 101 g
A) 9.61 × 10-2 g
B) 5.87 × 10-1 g
C) 1.18 g
D) 4.77 × 10-2 g
E) 7.24 × 101 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
48
How many liters does 3.336 g of nitrogen dioxide occupy at 30.1°C and 2.1 atm of pressure? The value of R = 0.0821 L atm mol-1 K-1.
A) 0.075 L
B) 0.086 L
C) 0.86 L
D) 4.0 L
E) 40 L
A) 0.075 L
B) 0.086 L
C) 0.86 L
D) 4.0 L
E) 40 L

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
49
For a gas mixture
A) the partial pressures of the gases at equilibrium are equal.
B) the total pressure is equal to the maximum partial pressure.
C) the total pressure is equal to the sum of the partial pressures.
D) the lightest gas will have the lowest partial pressure.
E) the lightest gas will have the highest partial pressure.
A) the partial pressures of the gases at equilibrium are equal.
B) the total pressure is equal to the maximum partial pressure.
C) the total pressure is equal to the sum of the partial pressures.
D) the lightest gas will have the lowest partial pressure.
E) the lightest gas will have the highest partial pressure.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
50
Suppose that 1.00 gram of each of the three inert gases, Ne, Ar, Kr, are combined in a 1.00-L flask at 25.0°C. Determine the partial pressures of each of the three gases in the flask in torr. Ne Ar Kr Total
A) 1.21 torr 0.612 torr 0.292 torr 2.12 torr
B) 24.4 torr 24.4 torr 24.4 torr 73.2 torr
C) 922 torr 466 torr 222 torr 923 torr
D) 922 torr 466 torr 222 torr 1.61 × 103 torr
E) 1.86 × 104 torr 1.86 × 104 torr 1.86 × 104 torr 5.58 × 103 torr
A) 1.21 torr 0.612 torr 0.292 torr 2.12 torr
B) 24.4 torr 24.4 torr 24.4 torr 73.2 torr
C) 922 torr 466 torr 222 torr 923 torr
D) 922 torr 466 torr 222 torr 1.61 × 103 torr
E) 1.86 × 104 torr 1.86 × 104 torr 1.86 × 104 torr 5.58 × 103 torr

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
51
Which is not a greenhouse gas?
A) carbon dioxide
B) methane
C) ozone
D) water vapor
E) nitrogen
A) carbon dioxide
B) methane
C) ozone
D) water vapor
E) nitrogen

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
52
Solid potassium chlorate can be decomposed to produce potassium chloride and oxygen according to the following equation. 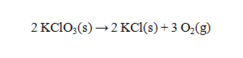 How many grams of KClO3 are needed to produce 752 mL of oxygen at 23°C and 0.995 atm, if the oxygen is collected over water? The vapor pressure of water at this temperature is 21.07 torr.
How many grams of KClO3 are needed to produce 752 mL of oxygen at 23°C and 0.995 atm, if the oxygen is collected over water? The vapor pressure of water at this temperature is 21.07 torr.
A) 0.0308 g
B) 2.43 g
C) 2.52 g
D) 3.66 g
E) 3.77 g
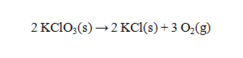 How many grams of KClO3 are needed to produce 752 mL of oxygen at 23°C and 0.995 atm, if the oxygen is collected over water? The vapor pressure of water at this temperature is 21.07 torr.
How many grams of KClO3 are needed to produce 752 mL of oxygen at 23°C and 0.995 atm, if the oxygen is collected over water? The vapor pressure of water at this temperature is 21.07 torr.A) 0.0308 g
B) 2.43 g
C) 2.52 g
D) 3.66 g
E) 3.77 g

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
53
If 7.63 g of an unknown gas occupies 79.1 mL at 25.02°C and 7.55 atm of pressure, what is the molar mass of the gas? The value of R = 0.0821 L atm mol-1 K-1.
A) 3.21 × 10-3 g/mol
B) 0.312 g/mol
C) 26.2 g/mol
D) 31.2 g/mol
E) 312 g/mol
A) 3.21 × 10-3 g/mol
B) 0.312 g/mol
C) 26.2 g/mol
D) 31.2 g/mol
E) 312 g/mol

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
54
What is the density (in g/L) of methane (CH4) at 25°C and 1.2 atm of pressure? The value of R = 0.0821 L atm mol-1 K-1.
A) 4.90 × 10-2 g/L
B) 1.20 g/L
C) 0.787 g/L
D) 9.9 g/L
E) 6.21 × 103 g/L
A) 4.90 × 10-2 g/L
B) 1.20 g/L
C) 0.787 g/L
D) 9.9 g/L
E) 6.21 × 103 g/L

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
55
What is the pressure of 2.50 mol of CO2 in a 1.00-L flask at 298K when calculated using the van der Waals equation? For CO2, the values of the van der Waals constants are a = 3.59 L2-atm/mol2 and b = 0.0427 L/mol.
A) 5.74
B) 46.0
C) 59.5
D) 61.1
E) 68.5
A) 5.74
B) 46.0
C) 59.5
D) 61.1
E) 68.5

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
56
A 4.35-g sample of gas has a density of 1.45 g/L when placed in a 3.00-L container at 21.0°C and 1.25 atm. What is a possible molecular formula of the gas? The value of R = 0.0821 L atm mol-1 K-1.
A) CH4
B) Cl2
C) CO
D) O2
E) NO
A) CH4
B) Cl2
C) CO
D) O2
E) NO

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
57
Which description of ideal gases and real gases is true?
A) The molecules in an ideal gas occupy more space than the molecules in a real gas.
B) The measured pressure for ideal gases is smaller than for real gases.
C) Gaseous atoms of helium and argon behave the same as real gases but differently as ideal gases.
D) The forces of attraction between molecules are greater in an ideal gas than in a real gas.
E) The van der Waals equation is the same as the ideal gas law in some cases.
A) The molecules in an ideal gas occupy more space than the molecules in a real gas.
B) The measured pressure for ideal gases is smaller than for real gases.
C) Gaseous atoms of helium and argon behave the same as real gases but differently as ideal gases.
D) The forces of attraction between molecules are greater in an ideal gas than in a real gas.
E) The van der Waals equation is the same as the ideal gas law in some cases.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck


