Exam 10: Gases and the Atmosphere
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
Arrange the following gaseous substances in order of decreasing average molecular speed at 25°C. 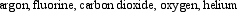
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
B
Based on temperature, there are four main layers to our atmosphere. What is the correct name for the layer in which we live?
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
A
Aluminum and oxygen react to form aluminum oxide. How many L of oxygen at 296 K and 1.4 atm of pressure are necessary to form 3.25 g of aluminum oxide? R = 0.0821 L atm mol-1 K-1.
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
C
If, at constant temperature, the volume of a flask equipped with a moveable piston is reduced to 1/4 its original volume, what must happen to the pressure?
(Multiple Choice)
4.9/5  (44)
(44)
When the external pressure on a balloon is decreased at constant temperature, what happens to the gas inside?
(Multiple Choice)
5.0/5  (34)
(34)
What is the correct name for the atmospheric layer in which weather phenomena occur?
(Multiple Choice)
4.9/5  (34)
(34)
A mixture of He, Ne and Ar has a total pressure of 0.80 atm and is found to contain 0.55 mol He, 0.94 mol Ne and 0.35 mol Ar. What is the partial pressure (in atm) of each gas? He Ne Ar
(Multiple Choice)
4.9/5  (32)
(32)
If, at constant pressure, the absolute temperature of a flask equipped with a moveable piston is reduced to half its original absolute temperature, what must be the new volume?
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the number of moles of a gas that occupy 7.3 × 103 mL at STP. The value of R = 0.0821 L atm mol-1 K-1.
(Multiple Choice)
4.7/5  (31)
(31)
Derive the gas constant (in L torr mol-1 K-1) given that 1.000 mole of a gas occupying 22.4 L at 0.00°C and 1.00 atm.
(Multiple Choice)
4.9/5  (39)
(39)
Calcium carbonate decomposes upon heating to form calcium oxide and carbon dioxide. A 1.75-g sample of calcium carbonate is placed in an empty, sealed 2.00-L steel container. The container is heated to 625°C and then allowed to cool to 25°C. What is the final pressure (in atm) inside the container? The value of R = 0.0821 L atm mol-1 K-1.
(Multiple Choice)
4.9/5  (26)
(26)
Which concept is not associated with the kinetic-molecular theory of gases?
(Multiple Choice)
4.8/5  (38)
(38)
What is the density of oxygen gas (O2) at STP? R = 0.0821 L atm mol-1 K-1.
(Multiple Choice)
4.8/5  (28)
(28)
What is the correct name for the atmospheric layer in which commercial jets fly?
(Multiple Choice)
4.9/5  (42)
(42)
A mixture of H2(g) and Cl2(g) exerts a total pressure of 2.50 atm. If the mole fraction of H2 is 0.490, what is the partial pressure (in atm) of H2?
(Multiple Choice)
4.9/5  (35)
(35)
Which description of the kinetic-molecular theory of gases is true?
(Multiple Choice)
4.8/5  (36)
(36)
Solid potassium chlorate can be decomposed to produce potassium chloride and oxygen according to the following equation. 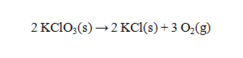 How many grams of KClO3 are needed to produce 752 mL of oxygen at 23°C and 0.995 atm, if the oxygen is collected over water? The vapor pressure of water at this temperature is 21.07 torr.
How many grams of KClO3 are needed to produce 752 mL of oxygen at 23°C and 0.995 atm, if the oxygen is collected over water? The vapor pressure of water at this temperature is 21.07 torr.
(Multiple Choice)
4.8/5  (28)
(28)
Showing 1 - 20 of 57
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)