Deck 2: Basic Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/57
Play
Full screen (f)
Deck 2: Basic Chemistry
1
Which of the following is/are an atom,an isotope and an ion?
A)H+
B)2H or deuterium
C)3H or tritium
D)H2 or hydrogen gas
E)All of the choices are atoms,isotopes and ions.
A)H+
B)2H or deuterium
C)3H or tritium
D)H2 or hydrogen gas
E)All of the choices are atoms,isotopes and ions.
A
2
In a water molecule,
A)the oxygen atom is more electronegative than the hydrogen atoms.
B)the oxygen atom has an overall negative charge with the hydrogen atoms having an overall positive charge.
C)unequal sharing of electrons results in a polar molecule.
D)All of the choices are correct.
A)the oxygen atom is more electronegative than the hydrogen atoms.
B)the oxygen atom has an overall negative charge with the hydrogen atoms having an overall positive charge.
C)unequal sharing of electrons results in a polar molecule.
D)All of the choices are correct.
D
3
Both 18O and 16O are found in nature.However,16O is the most common.Therefore,
A)these are different elements.
B)oxygen atoms can have eight or 10 neutrons.
C)18O has two additional electrons in its outer shell.
D)18O is the form of oxygen that provides living cells with life.
E)only the common form of 16O can bond with hydrogen atoms to form H2O.
A)these are different elements.
B)oxygen atoms can have eight or 10 neutrons.
C)18O has two additional electrons in its outer shell.
D)18O is the form of oxygen that provides living cells with life.
E)only the common form of 16O can bond with hydrogen atoms to form H2O.
B
4
Which of the following statements is NOT true about electron configurations?
A)If an atom has only one shell,it is complete with two electrons.
B)If an atom has two or more shells,the octet rule applies.
C)If an atom has two or more shells,the outer shell is complete with eight electrons.
D)Atoms with more than eight electrons in the outer shell react by gaining electrons.
E)Atoms with eight electrons in the outer shell are not reactive at all.
A)If an atom has only one shell,it is complete with two electrons.
B)If an atom has two or more shells,the octet rule applies.
C)If an atom has two or more shells,the outer shell is complete with eight electrons.
D)Atoms with more than eight electrons in the outer shell react by gaining electrons.
E)Atoms with eight electrons in the outer shell are not reactive at all.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
5
To determine the age of fairly recent fossils and organic artifacts,it is possible to analyze the amounts of the isotopes 14C and 14N,because over time the 14C-which originated in the atmosphere-breaks down into 14N.What net change occurred for this to happen?
A)The 14C lost an electron.
B)The 14C gained an electron.
C)The 14C lost a proton.
D)The 14C gained a proton.
E)The 14C gained a neutron.
A)The 14C lost an electron.
B)The 14C gained an electron.
C)The 14C lost a proton.
D)The 14C gained a proton.
E)The 14C gained a neutron.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
6
A research article indicates that researchers have used an isotope 3H to trace a certain metabolic process.From the symbol that is given,we know this is a hydrogen isotope with
A)three protons.
B)three neutrons.
C)three electrons.
D)one proton and two neutrons.
E)two protons and one neutron.
A)three protons.
B)three neutrons.
C)three electrons.
D)one proton and two neutrons.
E)two protons and one neutron.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following elements is NOT one of the six most common elements in living organisms?
A)carbon
B)oxygen
C)iron
D)nitrogen
E)hydrogen
A)carbon
B)oxygen
C)iron
D)nitrogen
E)hydrogen

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement is NOT true about subatomic particles?
A)Protons are found in the nucleus.
B)Neutrons have no electrical charge.
C)Electrons contain much less mass than neutrons.
D)Electrons are found in orbitals around the nucleus.
E)All electrons in an atom contain the same amount of energy.
A)Protons are found in the nucleus.
B)Neutrons have no electrical charge.
C)Electrons contain much less mass than neutrons.
D)Electrons are found in orbitals around the nucleus.
E)All electrons in an atom contain the same amount of energy.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
9
Which statement is NOT true about polar covalent bonds?
A)Most covalent bonds are nonpolar,with electrons shared fairly equally between the atoms.
B)Polar covalent bonds are important in the characteristics of water.
C)Electrons are shared unequally in a polar covalent bond.
D)The larger atom in a polar bond attracts the electron more strongly than the smaller atom.
E)The oxygen of a water molecule is electropositive relative to the hydrogen.
A)Most covalent bonds are nonpolar,with electrons shared fairly equally between the atoms.
B)Polar covalent bonds are important in the characteristics of water.
C)Electrons are shared unequally in a polar covalent bond.
D)The larger atom in a polar bond attracts the electron more strongly than the smaller atom.
E)The oxygen of a water molecule is electropositive relative to the hydrogen.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
10
Which statement is NOT true about ionic bonds?
A)One atom acts as an electron donor and another atom acts as an electron acceptor.
B)Electrons are completely lost or gained in ion formation.
C)An ion has the same number of electrons as a nonionic atom of the same element.
D)An ionic bond occurs between positive ions and negative ions.
E)A salt such as NaCl is formed by an ionic reaction.
A)One atom acts as an electron donor and another atom acts as an electron acceptor.
B)Electrons are completely lost or gained in ion formation.
C)An ion has the same number of electrons as a nonionic atom of the same element.
D)An ionic bond occurs between positive ions and negative ions.
E)A salt such as NaCl is formed by an ionic reaction.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
11
Which statement is NOT true about covalent bonds?
A)Covalent bonds form when an electron is completely lost or gained from an atom.
B)A covalent molecule contains one or more covalent bonds.
C)A single covalent bond is drawn as a line between two atoms.
D)A pair of electrons is shared between two atoms for each covalent bond.
E)Shared electrons allow an atom to complete its outer electron shell in a covalent molecule.
A)Covalent bonds form when an electron is completely lost or gained from an atom.
B)A covalent molecule contains one or more covalent bonds.
C)A single covalent bond is drawn as a line between two atoms.
D)A pair of electrons is shared between two atoms for each covalent bond.
E)Shared electrons allow an atom to complete its outer electron shell in a covalent molecule.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following elements would be more reactive with other elements?
A)boron,#5
B)neon,#10
C)argon,#18
D)helium,#2
A)boron,#5
B)neon,#10
C)argon,#18
D)helium,#2

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
13
If the atomic number of an element is 6 and the atomic mass is 12.01,how many protons are there in the nucleus?
A)12
B)6
C)24
D)52
A)12
B)6
C)24
D)52

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
14
An atom's atomic mass is best described as the mass of
A)the protons it contains.
B)the neutrons it contains.
C)electrons in the outermost shell.
D)protons and neutrons it contains.
E)protons and electrons it contains.
A)the protons it contains.
B)the neutrons it contains.
C)electrons in the outermost shell.
D)protons and neutrons it contains.
E)protons and electrons it contains.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following would be a proposed mechanism by which stomach antacids work?
A)Antacids dilute the solution,therefore lowering the pH.
B)Antacids are bases and by definition can absorb H+ out of a solution.
C)Antacids are bases and by definition can absorb OH- out of a solution.
D)Antacids contain mostly water and so they neutralize the solution.
A)Antacids dilute the solution,therefore lowering the pH.
B)Antacids are bases and by definition can absorb H+ out of a solution.
C)Antacids are bases and by definition can absorb OH- out of a solution.
D)Antacids contain mostly water and so they neutralize the solution.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
16
If you place the corner of a paper towel into a droplet of water the water moves across the paper towel.Which of the following would explain the movement of the water?
A)surface tension
B)cohesion
C)adhesion
D)both cohesion and adhesion
A)surface tension
B)cohesion
C)adhesion
D)both cohesion and adhesion

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
17
An orbital is best described as
A)the electron shell closest to the nucleus.
B)the outermost electron shell of an atom.
C)the volume of space in which electrons are most often found.
D)the original energy level of electrons in photosynthesis.
A)the electron shell closest to the nucleus.
B)the outermost electron shell of an atom.
C)the volume of space in which electrons are most often found.
D)the original energy level of electrons in photosynthesis.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
18
Figure: 
From the above table of radioisotopes and their properties,it is obvious that
A)the longer the half-life,the more energy emitted by the particles.
B)the longer the half-life,the less energy emitted by the particles.
C)radioisotopes of the same element must emit the same amount of energy in their emissions and decay at the same rate.
D)adjusted for time,radioisotopes emit the same amount of energy in their emissions.
E)energy and half-life are not directly related.

From the above table of radioisotopes and their properties,it is obvious that
A)the longer the half-life,the more energy emitted by the particles.
B)the longer the half-life,the less energy emitted by the particles.
C)radioisotopes of the same element must emit the same amount of energy in their emissions and decay at the same rate.
D)adjusted for time,radioisotopes emit the same amount of energy in their emissions.
E)energy and half-life are not directly related.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
19
Which is NOT true about the electrical charges in chemistry?
A)Protons carry a positive charge.
B)In an atom,the number of protons and neutrons must be equal.
C)An atom is neutral when the positive and negative charges balance.
D)An ion contains one or more positive or negative charges.
A)Protons carry a positive charge.
B)In an atom,the number of protons and neutrons must be equal.
C)An atom is neutral when the positive and negative charges balance.
D)An ion contains one or more positive or negative charges.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
20
Prior to prescription medications to control stomach acid and "heart burn" people consumed baking soda (sodium bicarbonate)to decrease their discomfort.This would indicate that sodium bicarbonate
A)effectively buffers stomach acid by releasing H+
B)should be sold as a prescription drug
C)blocks acid production by combining with OH-
D)neutralizes stomach acid by combining with excess H+
A)effectively buffers stomach acid by releasing H+
B)should be sold as a prescription drug
C)blocks acid production by combining with OH-
D)neutralizes stomach acid by combining with excess H+

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
21
Draw several (5 or 6)individual,unbonded water molecules.Simulate what happens when table salt (Na+Cl-)is added to water.Use the model you created to explain why salt is added to the roads in a 'snowy',cold climate.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
22
Anything below pH 7 is acidic and above pH 7 is basic.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
23
The blood buffer reactions described by H2CO3
 H+ + HCO3- indicates that
H+ + HCO3- indicates that
A)scientists are uncertain which direction the equation flows.
B)the reaction can flow either direction depending on whether there is an excess of hydrogen or hydroxide ions.
C)any reaction in one direction causes an immediate reverse reaction.
D)chemicals can swing wildly from acid to basic.
E)there is really no difference in chemistry whether a molecule is formed or dissociated.
 H+ + HCO3- indicates that
H+ + HCO3- indicates thatA)scientists are uncertain which direction the equation flows.
B)the reaction can flow either direction depending on whether there is an excess of hydrogen or hydroxide ions.
C)any reaction in one direction causes an immediate reverse reaction.
D)chemicals can swing wildly from acid to basic.
E)there is really no difference in chemistry whether a molecule is formed or dissociated.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
24
A coastal climate is moderated primarily by which of the following properties of water? Water
A)is the universal solvent.
B)is cohesive and adhesive.
C)has a high heat of evaporation.
D)has a high surface tension.
A)is the universal solvent.
B)is cohesive and adhesive.
C)has a high heat of evaporation.
D)has a high surface tension.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
25
A change of one pH unit represents a ten-fold increase or decrease in hydroxyl ion concentration.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
26
Classify the following substances as either hydrophobic or hydrophilic:



Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
27
Following nitrogen (78%)and oxygen (21%),argon is the next most common gas in the atmosphere (less than 1%).Checking the table of elements,you discover that argon is one of a family of atoms with outer shells already full of electrons.How is this related to the fact that these atoms have virtually no biological importance?

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
28
Draw the structural formula of a single water molecule.Note the location of partial positive and negative charges.Label the covalent bonds.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
29
Draw three water molecules and the hydrogen bonding that may occur between the molecules.Define hydrogen bonding and explain how and why it occurs.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
30
Figure:
-From the above table,it is apparent that:
A)triple bonds are stronger than double bonds; double bonds are stronger than single bonds.
B)triple bonds are weaker than double bonds; double bonds are weaker than single bonds.
C)carbon bonds are stronger than other bonds; hydrogen bonds are always weakest.
D)carbon forms only single bonds.
-From the above table,it is apparent that:
A)triple bonds are stronger than double bonds; double bonds are stronger than single bonds.
B)triple bonds are weaker than double bonds; double bonds are weaker than single bonds.
C)carbon bonds are stronger than other bonds; hydrogen bonds are always weakest.
D)carbon forms only single bonds.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
31
 Study the chart to determine the relationship between H+ concentration and pH.If you were to create a herbal remedy to decrease excess stomach acid,would you create a solution with a relatively greater or lesser number of hydrogen ions.
Study the chart to determine the relationship between H+ concentration and pH.If you were to create a herbal remedy to decrease excess stomach acid,would you create a solution with a relatively greater or lesser number of hydrogen ions.
Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
32
As a solid,water floats.This means that
A)solid water is less dense than liquid water.
B)organisms in ponds,lakes,and reservoirs can survive under the ice cover.
C)this is due to hydrogen bonding changes.
D)All of the choices are correct.
A)solid water is less dense than liquid water.
B)organisms in ponds,lakes,and reservoirs can survive under the ice cover.
C)this is due to hydrogen bonding changes.
D)All of the choices are correct.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
33
The scale indicates the relative concentrations of hydrogen and hydroxyl ions in a solution.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
34
Human blood has a pH of about 7.4.This is
A)neutral.
B)very acidic.
C)slightly acidic.
D)slightly basic.
A)neutral.
B)very acidic.
C)slightly acidic.
D)slightly basic.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
35
An abandoned Indiana coal mine spoil bank contains chunks of pyrite minerals.Under constant erosion and weathering,the pyrites leech large amounts of sulfuric acid (H2SO4).The spoil banks are also mixed with large quantities of basic limestone and clay carbonates.What should occur over time?
A)The pH level will drop until all acid has washed out.
B)The pH level will remain at 7.0 because of constant washing with rain.
C)The pH level will remain at 7.0 because all acid will be immediately neutralized by bases.
D)The pH levels will be spotty and vary over time,first more acidic but drifting back toward 7.0.
E)Bases always dominate over acids.
A)The pH level will drop until all acid has washed out.
B)The pH level will remain at 7.0 because of constant washing with rain.
C)The pH level will remain at 7.0 because all acid will be immediately neutralized by bases.
D)The pH levels will be spotty and vary over time,first more acidic but drifting back toward 7.0.
E)Bases always dominate over acids.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
36
All of the following are examples of damage caused by acid deposition from rain EXCEPT
A)leaching of aluminum from the soil into lakes which results in the formation of toxic methyl mercury from mercury in the lake sediments
B)weakens trees in the forests and kills seedlings
C)increased agricultural yields
D)damage to marble and limestone monuments
A)leaching of aluminum from the soil into lakes which results in the formation of toxic methyl mercury from mercury in the lake sediments
B)weakens trees in the forests and kills seedlings
C)increased agricultural yields
D)damage to marble and limestone monuments

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
37
Study the figures to determine which is liquid water and which is frozen water (ice).Explain your answer and predict if the water in Figure 2 would float or sink in the water in Figure 1. 
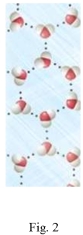



Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
38
The scale ranges from 1 to 15.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
39
The characteristic way in which atoms of an element react is most related to the
A)number of electrons in the outermost shell.
B)number of electrons in the innermost shell.
C)number of neutrons in the nucleus.
D)size of the nucleus.
A)number of electrons in the outermost shell.
B)number of electrons in the innermost shell.
C)number of neutrons in the nucleus.
D)size of the nucleus.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
40
pH 7 has a balanced level of H+ and OH-.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
41
What is the maximum number of electrons that will be in the 1st valence shell?
A)2
B)1
C)3
D)6
E)8
A)2
B)1
C)3
D)6
E)8

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
42
Which one is NOT one of the properties of water?
A)the frozen form is more dense than the liquid form
B)the frozen form is less dense than the liquid form
C)water is a solvent
D)water has a high heat capacity
E)water has a high heat of evaporation
A)the frozen form is more dense than the liquid form
B)the frozen form is less dense than the liquid form
C)water is a solvent
D)water has a high heat capacity
E)water has a high heat of evaporation

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
43
If an element contains 8 electrons how many electrons will be placed in the 2nd valence shell?
A)6
B)2
C)8
D)5
E)11
A)6
B)2
C)8
D)5
E)11

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
44
How many elements are required to form a molecule?
A)at least 2
B)at least 3
C)at least 4
D)at least 5
E)only 1
A)at least 2
B)at least 3
C)at least 4
D)at least 5
E)only 1

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
45
A solution with a pH of 6 has ________ times _________ OH- than a solution with a pH of 10.
A)40; more
B)4000; less
C)104; less
D)4; less
E)10-4 more
A)40; more
B)4000; less
C)104; less
D)4; less
E)10-4 more

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
46
The electrons are unequally shared in _______,and transferred in __________.
A)CH4, Na+Cl-
B)O2,CH4
C)Na+Cl-,H2O
D)H2O,N2
A)CH4, Na+Cl-
B)O2,CH4
C)Na+Cl-,H2O
D)H2O,N2

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
47
Use Bohr's model to draw a sodium (Na)atom and a chlorine (Cl)atom.Using your model,explain what happens when sodium reacts with chlorine to form table salt.Include in your explanation ion and ionic bond formation.Use your model to help you to decide whether NaCl is hydrophilic or hydrophobic. 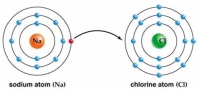
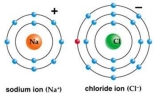



Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following concept circles best depicts the relationship between molecules and compounds (c = compound and m = molecule). A.
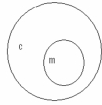 ?.
?.
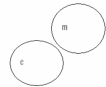 C.
C.
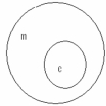
A)Option A
B)Option B
C)Option C Incorrect
 ?.
?. C.
C. 
A)Option A
B)Option B
C)Option C Incorrect

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
49
Which type of covalent bond is the strongest?
A)single
B)double
C)triple
D)quadruple
E)all covalent bonds are equal in strength
A)single
B)double
C)triple
D)quadruple
E)all covalent bonds are equal in strength

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
50
Which term refers to the attraction to water molecules?
A)hydrophilic
B)hydrophobic
C)hydrolysis
D)photolysis
E)nitrophylic
A)hydrophilic
B)hydrophobic
C)hydrolysis
D)photolysis
E)nitrophylic

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
51
Which property of water allows it to act as a transport medium?
A)cohesion
B)high heat of evaporation
C)high heat capacity
D)water is solvent
E)the frozen form is less dense than the liquid form
A)cohesion
B)high heat of evaporation
C)high heat capacity
D)water is solvent
E)the frozen form is less dense than the liquid form

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
52
Draw two hydrogen atoms using Bohr's model.Now bond them to form a molecule of hydrogen gas.Write the molecular formula.Explain what type of bond you've created and why this is a stable situation.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
53
Which substances are on the basic side of the pH scale?
A)baking soda,oven cleaner & human blood
B)baking soda,oven cleaner & urine
C)tomatoes,oven cleaner & human blood
D)beer,vinegar & black coffee
E)Great Salt Lake,oven cleaner,tears
A)baking soda,oven cleaner & human blood
B)baking soda,oven cleaner & urine
C)tomatoes,oven cleaner & human blood
D)beer,vinegar & black coffee
E)Great Salt Lake,oven cleaner,tears

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
54
The mass number refers to the number of ______ & ______ within an element.
A)protons & neutrons
B)protons & electrons
C)electrons & neutrons
D)protons & molecules
E)electrons & atoms
A)protons & neutrons
B)protons & electrons
C)electrons & neutrons
D)protons & molecules
E)electrons & atoms

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
55
A solution with a pH of 7.0 has _______ times ________ H+ than a solution of pH 10.
A)30; more
B)300; less
C)103; more
D)10-3; less
E)none of these are correct.
A)30; more
B)300; less
C)103; more
D)10-3; less
E)none of these are correct.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
56
All living things are 70 - 90% water.Use this graph to explain what characteristics of water protect living organisms from rapid temperature changes. 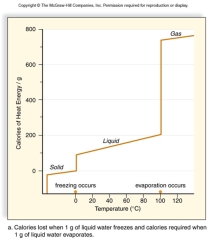


Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
57
This system of chemicals, 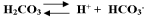 ,act as a buffer in the blood.If hydrogen ions are added to blood which of the following reactions would occur?
,act as a buffer in the blood.If hydrogen ions are added to blood which of the following reactions would occur?
A)
B)
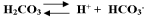 ,act as a buffer in the blood.If hydrogen ions are added to blood which of the following reactions would occur?
,act as a buffer in the blood.If hydrogen ions are added to blood which of the following reactions would occur?A)
B)

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck


