Deck 9: Chemical Bonds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/30
Play
Full screen (f)
Deck 9: Chemical Bonds
1
In an ionic compound, the metal
A)usually forms a negative ion.
B)takes the -ide ending.
C)has a Greek prefix like mono, di or tri.
D)is written first.
A)usually forms a negative ion.
B)takes the -ide ending.
C)has a Greek prefix like mono, di or tri.
D)is written first.
D
2
The formation of a positive ion
A)occurs when an atom gains a proton.
B)involves a release of energy.
C)occurs when an electron is removed from an atom.
D)occurs in covalent bonding.
A)occurs when an atom gains a proton.
B)involves a release of energy.
C)occurs when an electron is removed from an atom.
D)occurs in covalent bonding.
C
3
Ionic bonds occur between atoms from adjacent groups.
False
4
The breaking and making of chemical bonds can explain chemical reactions and energy flow.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
5
The subscript "2" in Mg(OH)2tells you that the charge of the hydroxide ion is (-2).

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
6
Atoms gain or lose electrons in order to attain a valence orbital arrangement like that of a noble gas.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
7
The amount of energy released when wood is burned is
A)greater than the amount of solar energy absorbed during its formation.
B)equal to the amount of solar energy absorbed during its formation.
C)less than the amount of solar energy absorbed during its formation.
D)greater or less than the amount of solar energy absorbed during its formation, depending on how it is burned.
A)greater than the amount of solar energy absorbed during its formation.
B)equal to the amount of solar energy absorbed during its formation.
C)less than the amount of solar energy absorbed during its formation.
D)greater or less than the amount of solar energy absorbed during its formation, depending on how it is burned.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
8
The formation of an ionic bond
A)involves a transfer of one or more electrons.
B)results in a release of energy.
C)helps atoms achieve a noble gas electron arrangement.
D)All of the above.
A)involves a transfer of one or more electrons.
B)results in a release of energy.
C)helps atoms achieve a noble gas electron arrangement.
D)All of the above.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
9
A covalent bond is a shared pair of electrons.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
10
What type of chemical bond does the sharing of a pair of electrons form?
A)covalent
B)ionic
C)metallic
D)double
A)covalent
B)ionic
C)metallic
D)double

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
11
An atom becomes a positive ion by gaining an electron.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
12
Ionic compounds are generally
A)white, crystalline solids.
B)gaseous substances.
C)syrupy liquids.
D)amorphous solids.
A)white, crystalline solids.
B)gaseous substances.
C)syrupy liquids.
D)amorphous solids.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
13
The representative elements have one to eight valence electrons.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
14
The -ide ending in nitrogen dioxide tells you that this is an ionic compound.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
15
Evidence of a chemical reaction includes
A)a color change.
B)a change in temperature.
C)the production of a gas.
D)All of the above.
A)a color change.
B)a change in temperature.
C)the production of a gas.
D)All of the above.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the electron dot diagram of the unknown element X:  This atom would most likely
This atom would most likely
A)form an ion of +6 charge.
B)react with hydrogen to form H2X.
C)lose two electrons when forming an ion.
D)form an ion of +2 charge.
 This atom would most likely
This atom would most likelyA)form an ion of +6 charge.
B)react with hydrogen to form H2X.
C)lose two electrons when forming an ion.
D)form an ion of +2 charge.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
17
Atoms gain or lose electrons in chemical reactions in order to increase their energy state.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
18
The smallest unit of a covalent compound that still retains the properties of the compound is called a (an)
A)electron.
B)atom.
C)molecule.
D)dipole.
A)electron.
B)atom.
C)molecule.
D)dipole.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
19
Three hydroxide ions are needed to form a neutral ionic compound with an aluminum ion.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
20
Atoms that have eight valence electrons would tend to
A)be very reactive.
B)be inert.
C)form positive ions.
D)form negative ions.
A)be very reactive.
B)be inert.
C)form positive ions.
D)form negative ions.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
21
The element M forms a stable ionic compound MCl2.If M were allowed to react with bromine, the resulting compound would have the formula
A)MBr.
B)M2Br.
C)MBr2.
D)there is not enough information to tell for sure.
A)MBr.
B)M2Br.
C)MBr2.
D)there is not enough information to tell for sure.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
22
What is the correct name for the ionic compound, CaF2?
A)calcium fluorine
B)calcium fluoride
C)calcium difluoride
D)monocalcium difluoride
A)calcium fluorine
B)calcium fluoride
C)calcium difluoride
D)monocalcium difluoride

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
23
When atoms of non-metallic elements react with one another, they tend to seek stability by
A)acquiring a noble gas electron arrangement.
B)losing electrons.
C)forming ionic bonds.
D)None of the above.
A)acquiring a noble gas electron arrangement.
B)losing electrons.
C)forming ionic bonds.
D)None of the above.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
24
What is the correct name for the compound, CuCl2?
A)copper chloride
B)copper dichloride
C)copper(II) chloride
D)copper chloride(II)
A)copper chloride
B)copper dichloride
C)copper(II) chloride
D)copper chloride(II)

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
25
Which combination of elements results in the formation of a white crystalline solid that dissolves to form a solution that conducts electricity?
A)metal and metal
B)non-metal and non-metal
C)metal and non-metal
D)metal and metalloid
A)metal and metal
B)non-metal and non-metal
C)metal and non-metal
D)metal and metalloid

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
26
Atoms from an element in group VA are allowed to react with atoms from an element in group VIIIA.What type of compound is likely to form?
A)ionic
B)covalent
C)polar
D)None of the above.
A)ionic
B)covalent
C)polar
D)None of the above.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
27
What is the formula when atoms of M and X (pictured below) react to form a stable ionic compound? 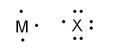
A)MX3
B)M3X
C)MX
D)MX5
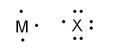
A)MX3
B)M3X
C)MX
D)MX5

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
28
What kind(s) of bonding takes place in NaOH?
A)covalent
B)ionic
C)metallic
D)both covalent and ionic
A)covalent
B)ionic
C)metallic
D)both covalent and ionic

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
29
A chemical bond that involves somewhat unequal sharing of electrons is called
A)ionic.
B)covalent.
C)polar.
D)coordinate.
A)ionic.
B)covalent.
C)polar.
D)coordinate.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
30
Atoms from an element in group IIA are allowed to react with atoms from an element in group VIIA.What type of compound is likely to form?
A)ionic
B)covalent
C)polar
D)None of the above.
A)ionic
B)covalent
C)polar
D)None of the above.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck


