Exam 9: Chemical Bonds
Exam 1: What Is Science30 Questions
Exam 2: Motion30 Questions
Exam 3: Energy30 Questions
Exam 4: Heat and Temperature30 Questions
Exam 5: Wave Motions and Sound30 Questions
Exam 6: Electricity30 Questions
Exam 7: Light35 Questions
Exam 8: Atoms and Periodic Properties30 Questions
Exam 9: Chemical Bonds30 Questions
Exam 10: Chemical Reactions30 Questions
Exam 11: Water and Solutions30 Questions
Exam 12: Organic Chemistry30 Questions
Exam 13: Nuclear Reactions30 Questions
Exam 14: The Universe30 Questions
Exam 15: The Solar System30 Questions
Exam 16: Earth in Space30 Questions
Exam 17: Rocks and Minerals30 Questions
Exam 18: Plate Tectonics30 Questions
Exam 19: Building Earths Surface30 Questions
Exam 20: Shaping Earths Surface30 Questions
Exam 21: Geologic Time30 Questions
Exam 22: The Atmosphere of Earth29 Questions
Exam 23: Weather and Climate30 Questions
Exam 24: Earths Waters30 Questions
Select questions type
Atoms from an element in group VA are allowed to react with atoms from an element in group VIIIA.What type of compound is likely to form?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
D
What is the correct name for the ionic compound, CaF2?
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
B
Atoms gain or lose electrons in order to attain a valence orbital arrangement like that of a noble gas.
Free
(True/False)
4.8/5  (41)
(41)
Correct Answer:
True
The subscript "2" in Mg(OH)2tells you that the charge of the hydroxide ion is (-2).
(True/False)
4.8/5  (33)
(33)
The breaking and making of chemical bonds can explain chemical reactions and energy flow.
(True/False)
4.8/5  (43)
(43)
The element M forms a stable ionic compound MCl2.If M were allowed to react with bromine, the resulting compound would have the formula
(Multiple Choice)
4.8/5  (35)
(35)
Three hydroxide ions are needed to form a neutral ionic compound with an aluminum ion.
(True/False)
4.8/5  (32)
(32)
The -ide ending in nitrogen dioxide tells you that this is an ionic compound.
(True/False)
4.8/5  (36)
(36)
A chemical bond that involves somewhat unequal sharing of electrons is called
(Multiple Choice)
5.0/5  (43)
(43)
What type of chemical bond does the sharing of a pair of electrons form?
(Multiple Choice)
4.7/5  (33)
(33)
The representative elements have one to eight valence electrons.
(True/False)
4.8/5  (31)
(31)
Atoms gain or lose electrons in chemical reactions in order to increase their energy state.
(True/False)
4.9/5  (30)
(30)
What is the formula when atoms of M and X (pictured below) react to form a stable ionic compound? 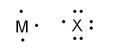
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 30
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)