Deck 12: Intermolecular Forces and Liquids and Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/138
Play
Full screen (f)
Deck 12: Intermolecular Forces and Liquids and Solids
1
Which of the following would be expected to have the highest vapor pressure at room temperature?
A)ethanol, bp = 78°C
B)methanol, bp = 65°C
C)water, bp = 100°C
D)acetone, bp = 56°C
A)ethanol, bp = 78°C
B)methanol, bp = 65°C
C)water, bp = 100°C
D)acetone, bp = 56°C
acetone, bp = 56°C
2
For which of the following species are the intermolecular interactions entirely due to dispersion forces?
A)C2H6
B)CH3OCH3
C)NO2
D)H2S
E)CaNO3
A)C2H6
B)CH3OCH3
C)NO2
D)H2S
E)CaNO3
C2H6
3
Which of the following substances should have the lowest boiling point?
A)CBr4
B)CBr3F
C)CBr2F2
D)CBrF3
E)CF4
A)CBr4
B)CBr3F
C)CBr2F2
D)CBrF3
E)CF4
CF4
4
Which one of the following substances is expected to have the highest boiling point?
A)HBr
B)HCl
C)HF
D)HI
A)HBr
B)HCl
C)HF
D)HI

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
5
Which one of the following substances should exhibit hydrogen bonding in the liquid state?
A)PH3
B)H2
C)H2S
D)CH4
E)NH3
A)PH3
B)H2
C)H2S
D)CH4
E)NH3

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of the following substances is expected to have the lowest melting point?
A)BrI
B)CsI
C)LiI
D)NaI
E)RbI
A)BrI
B)CsI
C)LiI
D)NaI
E)RbI

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following substances should have the highest boiling point?
A)CH4
B)Cl2
C)Kr
D)CH3Cl
E)N2
A)CH4
B)Cl2
C)Kr
D)CH3Cl
E)N2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following properties indicates the presence of strong intermolecular forces in a liquid?
A)a low heat of vaporization
B)a low critical temperature
C)a low vapor pressure
D)a low boiling point
E)None of the above.
A)a low heat of vaporization
B)a low critical temperature
C)a low vapor pressure
D)a low boiling point
E)None of the above.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following liquids would have the lowest viscosity at 25°C?
A)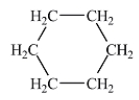
B)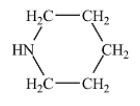
C)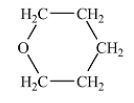
D)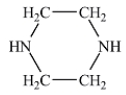
E)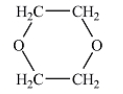
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
10
Helium atoms do not combine to form He2 molecules, yet He atoms do attract one another weakly through
A)dipole-dipole forces.
B)ion-dipole forces.
C)dispersion forces.
D)dipole-induced dipole forces.
E)hydrogen bonding.
A)dipole-dipole forces.
B)ion-dipole forces.
C)dispersion forces.
D)dipole-induced dipole forces.
E)hydrogen bonding.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
11
Atoms share electrons.
A)1 and 4
B)1 and 3
C)2 and 3
D)2 and 4
E)3 and 4
A)1 and 4
B)1 and 3
C)2 and 3
D)2 and 4
E)3 and 4

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
12
The molecular property related to the ease with which the electron density in a neutral atom or molecule can be distorted is called
A)a dipole moment.
B)polarizability.
C)a dispersion force.
D)surface tension.
E)a van der Waals force.
A)a dipole moment.
B)polarizability.
C)a dispersion force.
D)surface tension.
E)a van der Waals force.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
13
Which one of the following substances is expected to have the highest boiling point?
A)Br2
B)Cl2
C)F2
D)I2
A)Br2
B)Cl2
C)F2
D)I2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following liquids would have the highest viscosity at 25°C?
A)CH3OCH3
B)CH2Cl2
C)C2H5OH
D)CH3Br
E)HOCH2CH2OH
A)CH3OCH3
B)CH2Cl2
C)C2H5OH
D)CH3Br
E)HOCH2CH2OH

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following characteristics indicates the presence of weak intermolecular forces in a liquid?
A)a low heat of vaporization
B)a high critical temperature
C)a low vapor pressure
D)a high boiling point
E)None of the above.
A)a low heat of vaporization
B)a high critical temperature
C)a low vapor pressure
D)a high boiling point
E)None of the above.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following substances will have both dispersion forces and dipole-dipole forces?
A)HCl
B)BCl3
C)Br2
D)H2
E)CO2
A)HCl
B)BCl3
C)Br2
D)H2
E)CO2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
17
Each of the following substances is a liquid at -50°C. Place these liquids in order of increasing vapor pressure: dimethyl ether (CH3OCH3), propane (C3H8), and ethanol (CH3CH2OH).
A)ethanol < propane < dimethyl ether
B)ethanol < dimethyl ether < propane
C)propane < dimethyl ether < ethanol
D)dimethyl ether < ethanol < propane
E)propane < ethanol < dimethyl ether
A)ethanol < propane < dimethyl ether
B)ethanol < dimethyl ether < propane
C)propane < dimethyl ether < ethanol
D)dimethyl ether < ethanol < propane
E)propane < ethanol < dimethyl ether

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
18
For which of the following species are the dispersion forces strongest?
A)C4H10
B)C5H12
C)C6H14
D)C7H16
E)C8H18
A)C4H10
B)C5H12
C)C6H14
D)C7H16
E)C8H18

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following would be expected to have the lowest vapor pressure at room temperature?
A)ethanol, bp = 78°C
B)methanol, bp = 65°C
C)water, bp = 100°C
D)acetone, bp = 56°C
A)ethanol, bp = 78°C
B)methanol, bp = 65°C
C)water, bp = 100°C
D)acetone, bp = 56°C

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following properties indicates the presence of weak intermolecular forces in a liquid?
A)a high heat of vaporization
B)a high critical temperature
C)a high vapor pressure
D)a high boiling point
E)None of the above.
A)a high heat of vaporization
B)a high critical temperature
C)a high vapor pressure
D)a high boiling point
E)None of the above.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
21
Given the following liquids and their boiling points, which has the highest vapor pressure at its normal boiling point?
A)ethanol, bp = 78°C
B)methanol, bp = 65°C
C)water, bp = 100°C
D)benzene, bp = 80°C
E)The vapor pressure of each of the liquids at its normal boiling point would be the same.
A)ethanol, bp = 78°C
B)methanol, bp = 65°C
C)water, bp = 100°C
D)benzene, bp = 80°C
E)The vapor pressure of each of the liquids at its normal boiling point would be the same.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following atoms does not participate in hydrogen bonding?
A)S
B)O
C)F
D)N
A)S
B)O
C)F
D)N

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
23
The number of atoms in a body-centered cubic unit cell is
A)1.
B)2.
C)3.
D)4.
E)8.
A)1.
B)2.
C)3.
D)4.
E)8.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
24
Arrange the following in order of increasing boiling point: RbCl, CH3Cl, CH3OH, CH4.
A)CH3OH < CH3Cl < RbCl < CH4
B)CH3OH < CH4 < CH3Cl < RbCl
C)RbCl < CH3Cl < CH3OH < CH4
D)CH4 < CH3OH < CH3Cl < RbCl
E)CH4 < CH3Cl < CH3OH < RbCl
A)CH3OH < CH3Cl < RbCl < CH4
B)CH3OH < CH4 < CH3Cl < RbCl
C)RbCl < CH3Cl < CH3OH < CH4
D)CH4 < CH3OH < CH3Cl < RbCl
E)CH4 < CH3Cl < CH3OH < RbCl

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
25
The number of atoms in a face-centered cubic unit cell is
A)1.
B)2.
C)3.
D)4.
E)8.
A)1.
B)2.
C)3.
D)4.
E)8.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
26
Which one of the following crystallizes in a metallic lattice?
A)C
B)NaMnO4
C)K
D)LiClO4
E)K2Cr2O7
A)C
B)NaMnO4
C)K
D)LiClO4
E)K2Cr2O7

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following substances would have the highest critical temperature?
A)CH3Cl
B)C2H6
C)F2
D)H2
E)CO2
A)CH3Cl
B)C2H6
C)F2
D)H2
E)CO2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
28
Which one of the following substances crystallizes as a covalent crystal?
A)CaO
B)SiO2
C)CO2
D)Pb
E)KMnO4
A)CaO
B)SiO2
C)CO2
D)Pb
E)KMnO4

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
29
Which one of the following is an example of a covalent network solid?
A)SiO2
B)K
C)I2
D)CaCl2
E)None of these.
A)SiO2
B)K
C)I2
D)CaCl2
E)None of these.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
30
Which property of water allows a razor blade to float on it without sinking?
A)viscosity
B)surface tension
C)density
D)specific heat
E)triple point
A)viscosity
B)surface tension
C)density
D)specific heat
E)triple point

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
31
Which one of the following substances crystallizes as a molecular solid?
A)KI
B)SiO2
C)Sn
D)CH3OH
E)Al2(SO4)3
A)KI
B)SiO2
C)Sn
D)CH3OH
E)Al2(SO4)3

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
32
An example of a covalent network solid is
A)diamond.
B)potassium.
C)iodine.
D)sodium chloride.
E)None of these.
A)diamond.
B)potassium.
C)iodine.
D)sodium chloride.
E)None of these.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
33
Each of the following substances is a gas at 25°C and 1 atmosphere pressure. Which one will liquefy most easily when compressed at a constant temperature?
A)F2
B)H2
C)HF
D)SiH4
E)Ar
A)F2
B)H2
C)HF
D)SiH4
E)Ar

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the responses includes all of the following that can form hydrogen bonds with water molecules? (1)Na+ (2)CH3COOH (3)C2H6 (4)CH3NH2
A)(1)and (2)
B)(1)and (3)
C)(2)and (3)
D)(2)and (4)
E)(3)and (4)
A)(1)and (2)
B)(1)and (3)
C)(2)and (3)
D)(2)and (4)
E)(3)and (4)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
35
The structural form of the element Ge closely resembles the structure of
A)C (diamond).
B)N (diatomic).
C)As (tetrahedral).
D)S (S8 ring).
E)Kr (monatomic).
A)C (diamond).
B)N (diatomic).
C)As (tetrahedral).
D)S (S8 ring).
E)Kr (monatomic).

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
36
Krypton has a higher melting point than argon because of its
A)hydrogen bonding.
B)stronger dispersion forces.
C)permanent dipole moment.
D)ionic bonds.
E)greater ionization energy.
A)hydrogen bonding.
B)stronger dispersion forces.
C)permanent dipole moment.
D)ionic bonds.
E)greater ionization energy.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is not true with regard to water?
A)Water has a high heat capacity.
B)Water has an unusually high boiling point.
C)Water can form hydrogen bonds.
D)Ice is more dense than liquid water.
E)Water is a polar molecule.
A)Water has a high heat capacity.
B)Water has an unusually high boiling point.
C)Water can form hydrogen bonds.
D)Ice is more dense than liquid water.
E)Water is a polar molecule.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following substances should exhibit hydrogen bonding in the liquid state?
A)SiH4
B)H2
C)H2S
D)CH4
E)CH3NH2
A)SiH4
B)H2
C)H2S
D)CH4
E)CH3NH2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
39
Choose the response that lists the member of each of the following pairs that has the higher boiling point. (I)H2O or KI (II)HF or HI (III)Cl2 or Br2
A)H2O, HF, and Cl2
B)KI, HF, and Br2
C)KI, HI, and Br2
D)H2O, HI, and Cl2
E)KI, HF, and Cl2
A)H2O, HF, and Cl2
B)KI, HF, and Br2
C)KI, HI, and Br2
D)H2O, HI, and Cl2
E)KI, HF, and Cl2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
40
Which one of the following substances should exhibit hydrogen bonding in the liquid state?
A)PH3
B)He
C)H2S
D)CH4
E)CH3OH
A)PH3
B)He
C)H2S
D)CH4
E)CH3OH

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
41
MgO has the same crystal structure as NaCl, face-centered cubic. How many oxide ions surround each Mg2+ ion as nearest neighbors?
A)4
B)6
C)8
D)10
E)12
A)4
B)6
C)8
D)10
E)12

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
42
Potassium bromide, KBr, crystallizes like NaCl in a face-centered lattice. The ionic radii of K+ and Br- ions are 133 pm and 195 pm, respectively. Assuming that all Br- ions are positioned in the face and corners of the unit cell, while the K+ ions are positioned along the edge alternating between anions, calculate the length of a unit cell edge.
A)230 pm
B)328 pm
C)523 pm
D)656 pm
E)780 pm
A)230 pm
B)328 pm
C)523 pm
D)656 pm
E)780 pm

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
43
Acetic acid has a heat of fusion of 10.8 kJ/mol and a heat of vaporization of 24.3 kJ/mol. What is the expected value for the heat of sublimation of acetic acid?
A)35.1 kJ/mol
B)-13.5 kJ/mol
C)+13.5 kJ/mol
D)-35.1 kJ/mol
E)Not enough information is given to answer the question.
A)35.1 kJ/mol
B)-13.5 kJ/mol
C)+13.5 kJ/mol
D)-35.1 kJ/mol
E)Not enough information is given to answer the question.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
44
The mineral manganosite, manganese(II)oxide, crystallizes in the rock salt structure (the face-centered structure adopted by NaCl)with a density of 5.365 g/cm3. Find the unit cell edge length of manganosite.
A)444.5 pm
B)352.8 pm
C)280.0 pm
D)368.2 pm
E)417.9 pm
A)444.5 pm
B)352.8 pm
C)280.0 pm
D)368.2 pm
E)417.9 pm

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
45
Use the graph of vapor pressure to determine the normal boiling point of CHCl3. 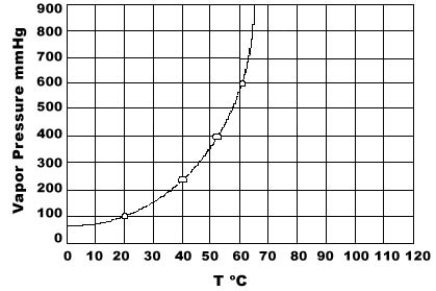
A)19°C
B)52°C
C)60°C
D)64°C
E)70°C

A)19°C
B)52°C
C)60°C
D)64°C
E)70°C

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
46
The specific heat of liquid ethanol, C2H5OH(l), is 2.46 J/g·°C and the heat of vaporization is 39.3 kJ/mol. The boiling point of ethanol is 78.3 °C. What amount of enthalpy is required to heat 50.0 g of liquid ethanol from 23.0 °C to ethanol vapor at 78.3 °C?
A)42.7 kJ
B)49.5 kJ
C)179 kJ
D)1970 kJ
E)6840 kJ
A)42.7 kJ
B)49.5 kJ
C)179 kJ
D)1970 kJ
E)6840 kJ

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
47
Use the graph of vapor pressure to determine the normal boiling point of O2. 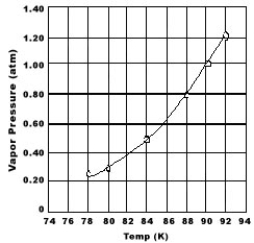
A)92 K
B)90 K
C)88 K
D)84 K
E)O2 doesn't boil because it is always a gas.

A)92 K
B)90 K
C)88 K
D)84 K
E)O2 doesn't boil because it is always a gas.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
48
The number of nearest neighbors (atoms that make contact)around each atom in a face-centered cubic lattice of a metal is
A)2.
B)4.
C)6.
D)8.
E)12.
A)2.
B)4.
C)6.
D)8.
E)12.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
49
Silver metal crystallizes in a face-centered cubic lattice with L as the length of one edge of the unit cube. The center-to-center distance between nearest silver atoms is
A)L/2.
B)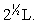
C)2L.
D)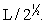
E)None of the above.
A)L/2.
B)
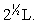
C)2L.
D)
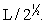
E)None of the above.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
50
Potassium crystallizes in a body-centered cubic lattice. How many atoms are there per unit cell?
A)1
B)2
C)4
D)6
E)8
A)1
B)2
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
51
Calculate the amount of heat needed to melt 2.00 kg of iron at its melting point (1,809 K), given that Hfus = 13.80 kJ/mol.
A)494 kJ
B)27,600 kJ
C)27.6 kJ
D)27,600 J
E)25,000 kJ
A)494 kJ
B)27,600 kJ
C)27.6 kJ
D)27,600 J
E)25,000 kJ

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
52
The zincblende structure of ZnS has the relatively large sulfide ions arranged at the lattice points of a face-centered cubic structure. The edge length of this cubic unit cell is 540.9 pm. Determine the density of zincblende.
A)3.081 g/cm3
B)1.023 g/cm3
C)4.091 g/cm3
D)2.046 g/cm3
E)2.032 g/cm3
A)3.081 g/cm3
B)1.023 g/cm3
C)4.091 g/cm3
D)2.046 g/cm3
E)2.032 g/cm3

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
53
Platinum has a face-centered cubic crystal structure and a density of 21.5 g/cm3. What is the radius of the platinum atom?
A)69 pm
B)98 pm
C)139 pm
D)196 pm
E)277 pm
A)69 pm
B)98 pm
C)139 pm
D)196 pm
E)277 pm

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is not an endothermic process?
A)melting of a solid
B)vaporization
C)raising the temperature of a gas
D)condensation of water vapor
E)sublimation of dry ice
A)melting of a solid
B)vaporization
C)raising the temperature of a gas
D)condensation of water vapor
E)sublimation of dry ice

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
55
The triple point of iodine is at 0.12 atm and 115°C. Thus, liquid I2
A)is more dense than I2 (s).
B)cannot exist above 115°C.
C)is liquid at room temperature.
D)cannot have a vapor pressure less than 91 torr.
A)is more dense than I2 (s).
B)cannot exist above 115°C.
C)is liquid at room temperature.
D)cannot have a vapor pressure less than 91 torr.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
56
Calculate the amount of heat that must be absorbed by 10.0 g of ice at -20°C to convert it to liquid water at 60.0°C. Given: specific heat (ice)= 2.1 J/g·°C; specific heat (water)= 4.18 J/g·°C; Hfus = 6.0 kJ/mol.
A)420 J
B)2,900 J
C)6,300 J
D)63 kJ
E)7.5 J
A)420 J
B)2,900 J
C)6,300 J
D)63 kJ
E)7.5 J

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
57
A liquid boils when its
A)vapor pressure is exactly 1 atmosphere.
B)vapor pressure is equal to, or greater than, the external pressure pushing on it.
C)temperature is equal to 273 K (standard temperature).
D)temperature is greater than room temperature.
A)vapor pressure is exactly 1 atmosphere.
B)vapor pressure is equal to, or greater than, the external pressure pushing on it.
C)temperature is equal to 273 K (standard temperature).
D)temperature is greater than room temperature.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
58
The heat capacity of liquid water is 4.18 J/g·°C and the heat of vaporization is 40.7 kJ/mol. How many kilojoules of heat must be provided to convert 1.00 g of liquid water at 67°C into 1.00 g of steam at 100°C?
A)22.7 kJ
B)40.8 kJ
C)2.2 kJ
D)2,400 J
E)40.8 J
A)22.7 kJ
B)40.8 kJ
C)2.2 kJ
D)2,400 J
E)40.8 J

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
59
Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.0 g/cm3 at 27°C. Calculate the atomic radius of Pd.
A)138 pm
B)1.95 * 10-8 nm
C)1.95 * 10-8 cm
D)154 pm
E)0.109 nm
A)138 pm
B)1.95 * 10-8 nm
C)1.95 * 10-8 cm
D)154 pm
E)0.109 nm

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
60
Vanadium crystallizes in a body-centered cubic lattice, and the length of the edge of a unit cell is 305 pm. What is the density of V?
A)5.96 g/cm3
B)2.98 g/cm3
C)2.98 * 10-6 g/cm3
D)5.96 * 10-30 g/cm3
E)11.9 g/cm3
A)5.96 g/cm3
B)2.98 g/cm3
C)2.98 * 10-6 g/cm3
D)5.96 * 10-30 g/cm3
E)11.9 g/cm3

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
61
Find the temperature at which water boils on a day in the mountains when the barometric pressure is 593 mmHg. (Given: the heat of vaporization of water is 40.79 kJ/mol)
A)93.1°C
B)117°C
C)41.5°C
D)97.0°C
E)68.1°C
A)93.1°C
B)117°C
C)41.5°C
D)97.0°C
E)68.1°C

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
62
What mass of water would need to evaporate from your skin in order to dissipate 1.7 * 105 J of heat from your body? H2O(l) H2O(g) Hvap = 40.7 kJ/mol
A)7.52 * 104 g
B)418 g
C)75.2 g
D)58.4 g
E)6.92 * 106 g
A)7.52 * 104 g
B)418 g
C)75.2 g
D)58.4 g
E)6.92 * 106 g

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
63
Find the temperature at which ethanol boils on a day in the mountains when the barometric pressure is 547 mmHg. (Given: The heat of vaporization of ethanol is 39.3 kJ/mol; the normal boiling point of ethanol is 78.3°C.)
A)76.5°C
B)69.9°C
C)10.0°C
D)77.9°C
E)74.6°C
A)76.5°C
B)69.9°C
C)10.0°C
D)77.9°C
E)74.6°C

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
64
Identify the dominant (strongest)type of intermolecular force present in H2S(g).

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
65
How much energy (heat)is required to convert 52.0 g of ice at -10.0°C to steam at 100°C? specific heat of ice: 2.09 J/g·°C Hfus = 6.02 kJ/mol
Specific heat of water: 4.18 J/g·°C Hvap = 40.7 kJ/mol
Specific heat of steam: 1.84 J/g·°C
A)2,570 kJ
B)1,086 kJ
C)157.8 kJ
D)40.2 kJ
E)22,957 kJ
Specific heat of water: 4.18 J/g·°C Hvap = 40.7 kJ/mol
Specific heat of steam: 1.84 J/g·°C
A)2,570 kJ
B)1,086 kJ
C)157.8 kJ
D)40.2 kJ
E)22,957 kJ

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
66
Solid iodine has a vapor pressure of 1.0 mmHg at 39°C. How many moles of iodine will sublime into a 500. mL flask at this temperature? If the volume of the flask is doubled at constant temperature, what will happen to the equilibrium vapor pressure of I2? (Assume some solid I2 is always present in the container.)
A)2.1 * 10-4 mol; vapor pressure increases
B)2.0 * 10-2 mol; vapor pressure increases
C)2.6 * 10-5 mol; no change in vapor pressure
D)2.1 * 10-4 mol; no change in vapor pressure
E)2.6* 10-5 mol; vapor pressure decreases
A)2.1 * 10-4 mol; vapor pressure increases
B)2.0 * 10-2 mol; vapor pressure increases
C)2.6 * 10-5 mol; no change in vapor pressure
D)2.1 * 10-4 mol; no change in vapor pressure
E)2.6* 10-5 mol; vapor pressure decreases

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
67
The normal boiling point of bromine is 58.8°C. Given that the vapor pressure of bromine is 75.0 torr at 2.5°C, calculate the molar enthalpy of vaporization of bromine.
A)3.76 kJ/mol
B)31.3 kJ/mol
C)2.90 kJ/mol
D)3.57 kJ/mol
E)29.7 kJ/mol
A)3.76 kJ/mol
B)31.3 kJ/mol
C)2.90 kJ/mol
D)3.57 kJ/mol
E)29.7 kJ/mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
68
The vapor pressure of a liquid in a closed container depends upon
A)the amount of liquid.
B)the surface area of the liquid.
C)the volume of the container.
D)the temperature.
E)None of the above.
A)the amount of liquid.
B)the surface area of the liquid.
C)the volume of the container.
D)the temperature.
E)None of the above.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
69
Use the following data to determine the molar heat of vaporization of chlorine. T (°C)-84.5 -71.2 -47.3
P (mmHg)40.0 100.0 400.0
A)34,700 J
B)21,900 J
C)317 J
D)712 J
E)9.99 kJ
P (mmHg)40.0 100.0 400.0
A)34,700 J
B)21,900 J
C)317 J
D)712 J
E)9.99 kJ

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
70
The molar heats of sublimation and fusion of iodine are 62.3 kJ/mol and15.3 kJ/mol, respectively. Calculate the molar heat of vaporization of liquid iodine.
A)77.6 kJ/mol
B)47.0 kJ/mol
C)-47.0 kJ/mol
D)-77.6 kJ/mol
E)4.07 kJ/mol
A)77.6 kJ/mol
B)47.0 kJ/mol
C)-47.0 kJ/mol
D)-77.6 kJ/mol
E)4.07 kJ/mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
71
Which one of the following elements would have the lowest melting point?
A)Kr
B)Br2
C)S8
D)Ca
E)K
A)Kr
B)Br2
C)S8
D)Ca
E)K

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following gases would have the highest critical temperature?
A)CH4
B)O2
C)CO2
D)NH3
E)Ne
A)CH4
B)O2
C)CO2
D)NH3
E)Ne

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
73
Identify the dominant (strongest)type of intermolecular force present in RbCl(s).

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
74
The vapor pressure of ethanol is 400 mmHg at 63.5°C. Its molar heat of vaporization is 39.3 kJ/mol. What is vapor pressure of ethanol, in mmHg, at 34.9°C?
A)1,510 mmHg
B)100 mmHg
C)200 mmHg
D)0.0099 mmHg
E)4.61 mmHg
A)1,510 mmHg
B)100 mmHg
C)200 mmHg
D)0.0099 mmHg
E)4.61 mmHg

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
75
The molar enthalpy of vaporization of carbon disulfide is 26.74 kJ/mol, and its normal boiling point is 46°C. What is the vapor pressure of CS2 at 0°C?
A)447 torr
B)4160 torr
C)313 torr
D)139 torr
E)5.47 torr
A)447 torr
B)4160 torr
C)313 torr
D)139 torr
E)5.47 torr

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
76
Based on the phase diagram shown below, how will the melting point of the substance change if the pressure is increased above 1 atm? 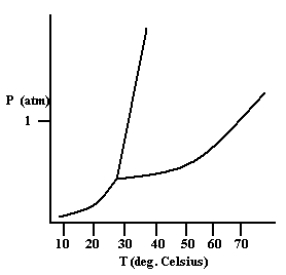
A)The melting point will decrease.
B)The melting point will remain the same.
C)The melting point will increase.
D)The substance will not melt at pressures of 1 atm and above; instead, the solid sublimes to form the gas phase.

A)The melting point will decrease.
B)The melting point will remain the same.
C)The melting point will increase.
D)The substance will not melt at pressures of 1 atm and above; instead, the solid sublimes to form the gas phase.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
77
The normal boiling point of methanol (CH3OH)is 64.6°C. Given that the vapor pressure of methanol is 75.0 torr at 15.2°C, calculate the molar enthalpy of vaporization of methanol.
A)0.383 kJ/mol
B)3.00 kJ/mol
C)38.0 kJ/mol
D)27.5 kJ/mol
E)74.7 kJ/mol
A)0.383 kJ/mol
B)3.00 kJ/mol
C)38.0 kJ/mol
D)27.5 kJ/mol
E)74.7 kJ/mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
78
The molar enthalpy of vaporization of hexane (C6H14)is 28.9 kJ/mol, and its normal boiling point is 68.73°C. What is the vapor pressure of hexane at 25°C?
A)171 torr
B)4.44 torr
C)117 torr
D)3370 torr
E)759 torr
A)171 torr
B)4.44 torr
C)117 torr
D)3370 torr
E)759 torr

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
79
The molar enthalpy of vaporization of boron tribromide is 30.5 kJ/mol, and its normal boiling point is 91°C. What is the vapor pressure of BBr3 at 20°C?
A)11.5 torr
B)311 torr
C)5.31 torr
D)143 torr
E)66.1 torr
A)11.5 torr
B)311 torr
C)5.31 torr
D)143 torr
E)66.1 torr

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
80
Octane is a liquid component of gasoline. Given the following vapor pressures of octane at various temperatures, estimate the boiling point of octane in Leadville, Colorado, where the atmospheric pressure is 496 mmHg. 400 mmHg @ 104°C, 500 mmHg @ 111°C, 600 mmHg @ 117°C,
700 mmHg @ 122°C, 760 mmHg @ 125°C
A)125°C
B)120°C
C)115°C
D)110°C
E)105°C
700 mmHg @ 122°C, 760 mmHg @ 125°C
A)125°C
B)120°C
C)115°C
D)110°C
E)105°C

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck


