Exam 12: Intermolecular Forces and Liquids and Solids
Exam 1: Introduction153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Stoichiometry168 Questions
Exam 4: Reactions in Aqueous Solution156 Questions
Exam 5: Gases109 Questions
Exam 6: Energy Relationships in Chemical Reactions105 Questions
Exam 7: The Electronic Structure of Atoms115 Questions
Exam 8: The Periodic Table119 Questions
Exam 9: Chemical Bonding I: the Covalent Bond118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals120 Questions
Exam 11: Introduction to Organic Chemistry57 Questions
Exam 12: Intermolecular Forces and Liquids and Solids138 Questions
Exam 13: Physical Properties of Solutions109 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium99 Questions
Exam 16: Acids and Bases163 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria92 Questions
Exam 18: Thermodynamics112 Questions
Exam 19: Redox Reactions and Electrochemistry138 Questions
Exam 20: The Chemistry of Coordination Compounds76 Questions
Exam 21: Nuclear Chemistry112 Questions
Exam 22: Organic Polymerssynthetic and Natural42 Questions
Select questions type
Which of the following substances should have the highest boiling point?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
D
Silver metal crystallizes in a face-centered cubic lattice with L as the length of one edge of the unit cube. The center-to-center distance between nearest silver atoms is
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
D
Indicate all the types of intermolecular forces of attraction in CH3OH(l).
Free
(Short Answer)
4.9/5  (40)
(40)
Correct Answer:
hydrogen bonding and dispersion
Suppose the atoms in a two-dimensional crystal have the following arrangement: 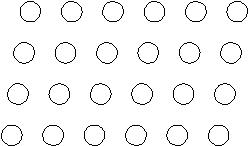 On the drawing above, sketch the unit cell of this crystal.
On the drawing above, sketch the unit cell of this crystal.
(Essay)
5.0/5  (38)
(38)
Which of the following would be expected to have the highest vapor pressure at room temperature?
(Multiple Choice)
4.8/5  (41)
(41)
MgO has the same crystal structure as NaCl, face-centered cubic. How many oxide ions surround each Mg2+ ion as nearest neighbors?
(Multiple Choice)
4.8/5  (40)
(40)
The normal boiling point of bromine is 58.8°C. Given that the vapor pressure of bromine is 75.0 torr at 2.5°C, calculate the molar enthalpy of vaporization of bromine.
(Multiple Choice)
4.9/5  (36)
(36)
Iron crystallizes in a body-centered cubic unit. The edge of this cell is 287 pm. Calculate the density of iron.
(Short Answer)
5.0/5  (24)
(24)
Of the given pair of compounds, which would have the higher boiling point?
C3H8 or CH3OCH3
(Short Answer)
4.8/5  (35)
(35)
The molar enthalpy of vaporization of carbon disulfide is 26.74 kJ/mol, and its normal boiling point is 46°C. What is the vapor pressure of CS2 at 0°C?
(Multiple Choice)
4.9/5  (45)
(45)
Of the given pair of compounds, which would have the higher boiling point?
NH3 or CH4
(Short Answer)
4.7/5  (36)
(36)
Crystals of elemental sulfur are easily crushed, and melt at 113°C. Liquid sulfur does not conduct electricity. What kind of crystal is this?
(Short Answer)
4.7/5  (31)
(31)
Which of the following atoms does not participate in hydrogen bonding?
(Multiple Choice)
4.8/5  (40)
(40)
Of the pair of compounds given, which would have the stronger intermolecular forces of attraction?
NH3 or PH3
(Short Answer)
5.0/5  (36)
(36)
Krypton has a higher melting point than argon because of its
(Multiple Choice)
4.9/5  (31)
(31)
Suppose the atoms in a two-dimensional crystal have the following arrangement: 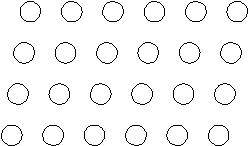 What is the coordination number of each atom in this crystal?
What is the coordination number of each atom in this crystal?
(Short Answer)
4.9/5  (36)
(36)
Given the following compound and its boiling point, identify whether it is polar or nonpolar: F2, -188.1°C.
(Short Answer)
4.7/5  (36)
(36)
Methane has a heat of fusion of 0.84 kJ/mol and a heat of vaporization of 9.2 kJ/mol. Estimate the value for the heat of sublimation.
(Short Answer)
4.9/5  (45)
(45)
The vapor pressure of ethanol is 400 mmHg at 63.5°C. Its molar heat of vaporization is 39.3 kJ/mol. What is vapor pressure of ethanol, in mmHg, at 34.9°C?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 138
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)