Deck 13: Physical Properties of Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/109
Play
Full screen (f)
Deck 13: Physical Properties of Solutions
1
What is the percent CsCl by mass in a 0.711 M CsCl solution that has a density of 1.091 g/mL?
A)3.87 * 10-4 %
B)3.87 * 10-1 %
C)11.0 %
D)1.10 %
E)6.50 * 10-2 %
A)3.87 * 10-4 %
B)3.87 * 10-1 %
C)11.0 %
D)1.10 %
E)6.50 * 10-2 %
11.0 %
2
Heat of solution
A)is never positive ( H°soln 0), because the solute-solvent attraction is never weaker than the combination of the solute-solute attraction and solvent-solvent attraction.
B)is always positive ( H°soln > 0), because the solute-solvent attraction is always weaker than the combination of the solute-solute attraction and solvent-solvent attraction.
C)is always zero ( H°soln = 0), because the solute-solvent attraction is defined as the average of the solute-solute attraction and solvent-solvent attraction.
D)is always negative ( H°soln < 0), because the solute-solvent attraction is always stronger than the combination of the solute-solute attraction and solvent-solvent attraction.
E)may be positive, zero, or negative, depending on the relative strength of the solute-solvent, solute-solute, and solvent-solvent attractive forces.
A)is never positive ( H°soln 0), because the solute-solvent attraction is never weaker than the combination of the solute-solute attraction and solvent-solvent attraction.
B)is always positive ( H°soln > 0), because the solute-solvent attraction is always weaker than the combination of the solute-solute attraction and solvent-solvent attraction.
C)is always zero ( H°soln = 0), because the solute-solvent attraction is defined as the average of the solute-solute attraction and solvent-solvent attraction.
D)is always negative ( H°soln < 0), because the solute-solvent attraction is always stronger than the combination of the solute-solute attraction and solvent-solvent attraction.
E)may be positive, zero, or negative, depending on the relative strength of the solute-solvent, solute-solute, and solvent-solvent attractive forces.
may be positive, zero, or negative, depending on the relative strength of the solute-solvent, solute-solute, and solvent-solvent attractive forces.
3
Which of the following gives the molarity of a 17.0 % by mass solution of sodium acetate, CH3COONa (molar mass = 82.0 g/mol)in water? The density of the solution is 1.09 g/mL.
A)2.26 * 10 - 6 M
B)0.207 M
C)2.07 M
D)2.26 M
E)2.72 M
A)2.26 * 10 - 6 M
B)0.207 M
C)2.07 M
D)2.26 M
E)2.72 M
2.26 M
4
Calculate the percent by mass of potassium nitrate in a solution made from 45.0 g KNO3 and 295 mL of water. The density of water is 0.997 g/mL.
A)1.51 %
B)7.57 %
C)13.3 %
D)15.2 %
E)None of these
A)1.51 %
B)7.57 %
C)13.3 %
D)15.2 %
E)None of these

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
5
Which response lists all the following pairs that are miscible liquids. Pair #1: octane (C8H18)and water
Pair #2: acetic acid (CH3COOH)and water
Pair #3: octane (C8H18)and carbon tetrachloride (CCl4)
A)1, 3
B)1, 2
C)3
D)2
E)2, 3
Pair #2: acetic acid (CH3COOH)and water
Pair #3: octane (C8H18)and carbon tetrachloride (CCl4)
A)1, 3
B)1, 2
C)3
D)2
E)2, 3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
6
Determine the mass percent HCl in a 1.2 M solution of hydrochloric acid with a density of 1.019 g/mL?
A)3.0 %
B)4.3 %
C)8.6 %
D)13%
E)30. %
A)3.0 %
B)4.3 %
C)8.6 %
D)13%
E)30. %

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
7
What is the percent CdSO4 by mass in a 1.0 molal aqueous CdSO4 solution?
A)1.00 * 10-3 %
B)0.100 %
C)17.2 %
D)20.8 %
E)24.4 %
A)1.00 * 10-3 %
B)0.100 %
C)17.2 %
D)20.8 %
E)24.4 %

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
8
A 20.0 % by mass solution of phosphoric acid (H3PO4)in water has a density of 1.114 g/mL at 20°C. What is the molarity of this solution?
A)0.0114 M
B)0.0568 M
C)0.114 M
D)2.27 M
E)11.4 M
A)0.0114 M
B)0.0568 M
C)0.114 M
D)2.27 M
E)11.4 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
9
In which of the following solvents would you expect KBr to be most soluble?
A)C6H14 (hexane)
B)CH3CH2OH (ethanol)
C)C6H6 (benzene)
D)CCl4 (carbon tetrachloride)
E)C6H12 (cyclohexane)
A)C6H14 (hexane)
B)CH3CH2OH (ethanol)
C)C6H6 (benzene)
D)CCl4 (carbon tetrachloride)
E)C6H12 (cyclohexane)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
10
What is the molarity of a solution of 10 % by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol)by mass? The density of the solution is 1.10 g/mL.
A)0.528 M
B)0.436 M
C)0.479 M
D)0.048 M
E)22.9 M
A)0.528 M
B)0.436 M
C)0.479 M
D)0.048 M
E)22.9 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following would be immiscible with water?
A)
B)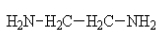
C)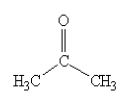
D)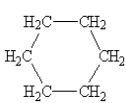
E)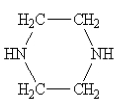
A)

B)
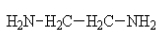
C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
12
In how many grams of water should 25.31 g of potassium nitrate (KNO3)be dissolved to prepare a 0.1982 m solution?
A)250.0 g
B)792.0 g
C)1,000. g
D)1,263 g
E)7,917 g
A)250.0 g
B)792.0 g
C)1,000. g
D)1,263 g
E)7,917 g

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
13
A 15.00 % by mass solution of lactose (C12H22O11, 342.30 g/mol)in water has a density of 1.0602 g/mL at 20°C. What is the molarity of this solution?
A)0.03097 M
B)0.4133 M
C)0.4646 M
D)1.590 M
E)3.097 M
A)0.03097 M
B)0.4133 M
C)0.4646 M
D)1.590 M
E)3.097 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
14
How many grams of water are needed to dissolve 27.8 g of ammonium nitrate NH4NO3 in order to prepare a 0.452 m solution?
A)769 g
B)36.2 g
C)100. g
D)0.157 g
E)157 g
A)769 g
B)36.2 g
C)100. g
D)0.157 g
E)157 g

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following liquids would make a good solvent for iodine, I2?
A)HCl
B)H2O
C)CH3OH
D)NH3
E)CS2
A)HCl
B)H2O
C)CH3OH
D)NH3
E)CS2

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
16
Calculate the molality of a solution containing 14.3 g of NaCl in 42.2 g of water.
A)2.45 * 10-4 m
B)5.80 * 10-4 m
C)2.45 * 10-1 m
D)103 m
E)5.80 m
A)2.45 * 10-4 m
B)5.80 * 10-4 m
C)2.45 * 10-1 m
D)103 m
E)5.80 m

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
17
What is the molarity of a solution that is 7.00 % by mass magnesium sulfate and has a density of 1.071 g/mL?
A)0.0890 M
B)0.496 M
C)0.543 M
D)0.623 M
E)1.32 M
A)0.0890 M
B)0.496 M
C)0.543 M
D)0.623 M
E)1.32 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
18
A 9.50 % by mass solution of acetone (C3H6O)in water has a density of 0.9849 g/mL at 20°C. What is the molarity of this solution?
A)0.621 M
B)1.61 M
C)1.66 M
D)1.71 M
E)16.9 M
A)0.621 M
B)1.61 M
C)1.66 M
D)1.71 M
E)16.9 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
19
A saturated solution
A)contains more solute than solvent.
B)contains more solvent than solute.
C)contains equal moles of solute and solvent.
D)contains the maximum amount of solute that will dissolve in that solvent at that temperature.
E)contains a solvent with only sigma bonds and no pi bonds (i.e. only single bonds, with no double or triple bonds).
A)contains more solute than solvent.
B)contains more solvent than solute.
C)contains equal moles of solute and solvent.
D)contains the maximum amount of solute that will dissolve in that solvent at that temperature.
E)contains a solvent with only sigma bonds and no pi bonds (i.e. only single bonds, with no double or triple bonds).

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following compounds should be soluble in CCl4?
A)NaCl
B)H2O
C)NaOH
D)C8H18
E)None of these
A)NaCl
B)H2O
C)NaOH
D)C8H18
E)None of these

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
21
The solubility of CO2 gas in water
A)increases with increasing gas pressure.
B)increases with decreasing gas pressure.
C)decreases with increasing gas pressure.
D)is not dependent on pressure.
A)increases with increasing gas pressure.
B)increases with decreasing gas pressure.
C)decreases with increasing gas pressure.
D)is not dependent on pressure.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
22
Calculate the molality of a 15.0% by mass solution of MgCl2 in H2O. The density of this solution is 1.127 g/mL.
A)0.157 m
B)11.8 m
C)1.86 m
D)0.0134 m
E)1.58 m
A)0.157 m
B)11.8 m
C)1.86 m
D)0.0134 m
E)1.58 m

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
23
Oxygen gas makes up 21 % of the atmosphere by volume. What is the solubility of O2(g)in water at 25°C if the atmospheric pressure is 741 mmHg? The Henry's law constant for oxygen gas at 25°C is 1.3 * 10-3 mol/L·atm.
A)2.7 * 10-4 M
B)1.3 * 10-3 M
C)6.2 * 10-3 M
D)9.6 * 10-3 M
E)0.96 M
A)2.7 * 10-4 M
B)1.3 * 10-3 M
C)6.2 * 10-3 M
D)9.6 * 10-3 M
E)0.96 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
24
The solubility of gases in water usually decreases with
A)increasing pressure.
B)increasing temperature.
C)decreasing temperature.
A)increasing pressure.
B)increasing temperature.
C)decreasing temperature.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
25
The solubility of nitrogen gas at 25°C and a nitrogen pressure of 522 mmHg is 4.7 * 10 - 4 mol/L. What is the value of the Henry's Law constant in mol/L·atm?
A)6.8 * 10-4 mol/L·atm
B)4.7 * 10-4 mol/L·atm
C)3.2 * 10-4 mol/L·atm
D)9.0 * 10-7 mol/L·atm
E)1.5 * 103 mol/L·atm
A)6.8 * 10-4 mol/L·atm
B)4.7 * 10-4 mol/L·atm
C)3.2 * 10-4 mol/L·atm
D)9.0 * 10-7 mol/L·atm
E)1.5 * 103 mol/L·atm

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
26
According to Raoult's law, which statement is false?
A)The vapor pressure of a solvent over a solution decreases as its mole fraction increases.
B)The solubility of a gas increases as the temperature decreases.
C)The vapor pressure of a solvent over a solution is less than that of pure solvent.
D)The greater the pressure of a gas over a solution the greater its solubility.
E)Ionic solutes dissociate in solution causing an enhancement of all colligative properties.
A)The vapor pressure of a solvent over a solution decreases as its mole fraction increases.
B)The solubility of a gas increases as the temperature decreases.
C)The vapor pressure of a solvent over a solution is less than that of pure solvent.
D)The greater the pressure of a gas over a solution the greater its solubility.
E)Ionic solutes dissociate in solution causing an enhancement of all colligative properties.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
27
What is the mass percent CH3OH of a 0.256 m CH3OH(aq)solution?
A)0.814 %
B)0.992 %
C)1.23 %
D)1.29 %
E)1.51 %
A)0.814 %
B)0.992 %
C)1.23 %
D)1.29 %
E)1.51 %

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
28
The density of a 20.3 M CH3OH (methanol)solution is 0.858 g/mL. What is the molality of this solution? H2O is the solvent.
A)17.4 m
B)20.8 m
C)23.7 m
D)70.0 m
E)97.6 m
A)17.4 m
B)20.8 m
C)23.7 m
D)70.0 m
E)97.6 m

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
29
A solution of carbon tetrachloride in benzene, C6H6, at 20°C has a total vapor pressure of 78.50 mmHg. (Assume that this solution is ideal.)The vapor pressure of pure benzene at this temperature is 74.61 mmHg and its density is 0.87865 g/cm3; the vapor pressure of pure carbon tetrachloride is 91.32 mmHg and its density is 1.5940 g/cm3. What percentage of the volume of this solution is due to carbon tetrachloride? (Hint: assume that you have 1.000 L of solution.)
A)75.2%
B)76.7%
C)14.3%
D)24.8%
E)23.3%
A)75.2%
B)76.7%
C)14.3%
D)24.8%
E)23.3%

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
30
Consider a solution made from a nonvolatile solute and a volatile solvent. Which statement is true?
A)The vapor pressure of the solution is always greater than the vapor pressure of the pure solvent.
B)The boiling point of the solution is always greater than the boiling point of the pure solvent.
C)The freezing point of the solution is always greater than the freezing point of the pure solvent.
A)The vapor pressure of the solution is always greater than the vapor pressure of the pure solvent.
B)The boiling point of the solution is always greater than the boiling point of the pure solvent.
C)The freezing point of the solution is always greater than the freezing point of the pure solvent.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following has the greater molal concentration (molality)?
A)1.0 m KNO3
B)1.0 M KNO3
C)Both have same molality.
A)1.0 m KNO3
B)1.0 M KNO3
C)Both have same molality.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate the molality of 6.0 M H2SO4 solution. The density of the solution is 1.34 g/mL.
A)4.48 m
B)7.98 m
C)8.10 m
D)8.43 m
E)10.2 m
A)4.48 m
B)7.98 m
C)8.10 m
D)8.43 m
E)10.2 m

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
33
The vapor pressure of water at 20°C is 17.5 mmHg. What is the vapor pressure of water over a solution prepared from 2.00 * 102 g of sucrose (C12H22O11)and 3.50 * 102 g water?
A)0.51 mmHg
B)16.0 mmHg
C)17.0 mmHg
D)18.0 mmHg
E)19.4 mmHg
A)0.51 mmHg
B)16.0 mmHg
C)17.0 mmHg
D)18.0 mmHg
E)19.4 mmHg

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
34
The solubility of oxygen in lakes high in the Rocky Mountains is affected by the altitude. If the solubility of O2 from the air is 2.67 * 10-4 M at sea level and 25°C, what is the solubility of O2 at an elevation of 12,000 ft where the atmospheric pressure is 0.657 atm? Assume the temperature is 25°C, and that the mole fraction of O2 in air is 0.209 at both 12,000 ft and at sea level.
A)1.75 * 10-4 M
B)2.67 * 10-4 M
C)3.66 * 10-5 M
D)4.06 * 10-4 M
E)None of the above.
A)1.75 * 10-4 M
B)2.67 * 10-4 M
C)3.66 * 10-5 M
D)4.06 * 10-4 M
E)None of the above.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
35
A solution of chloroform, CHCl3, and acetone, (CH3)2CO, exhibits a negative deviation from Raoult's law. This result implies that
A)chloroform-chloroform interactions are stronger than chloroform-acetone interactions.
B)chloroform-chloroform interactions are weaker than chloroform-acetone interactions.
C)acetone-acetone interactions are stronger than chloroform-acetone interactions.
D)acetone-acetone interactions are weaker than chloroform-acetone interactions.
E)Both B and D.
F)Both A and C.
A)chloroform-chloroform interactions are stronger than chloroform-acetone interactions.
B)chloroform-chloroform interactions are weaker than chloroform-acetone interactions.
C)acetone-acetone interactions are stronger than chloroform-acetone interactions.
D)acetone-acetone interactions are weaker than chloroform-acetone interactions.
E)Both B and D.
F)Both A and C.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
36
What is the molality of a solution that is 3.68 % by mass calcium chloride?
A)0.0332 m
B)0.332 m
C)0.344 m
D)0.464 m
E)0.506 m
A)0.0332 m
B)0.332 m
C)0.344 m
D)0.464 m
E)0.506 m

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
37
The solubility of CO2 gas in water
A)increases with increasing temperature.
B)decreases with decreasing temperature.
C)decreases with increasing temperature.
D)is not dependent on temperature.
A)increases with increasing temperature.
B)decreases with decreasing temperature.
C)decreases with increasing temperature.
D)is not dependent on temperature.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
38
A solution is 40.0% by volume benzene (C6H6)in carbon tetrachloride at 20°C. The vapor pressure of pure benzene at this temperature is 74.61 mmHg and its density is 0.87865 g/cm3; the vapor pressure of pure carbon tetrachloride is 91.32 mmHg and its density is 1.5940 g/cm3. If this solution is ideal, its total vapor pressure at 20°C is
A)84.64 mmHg.
B)84.30 mmHg.
C)82.96 mmHg.
D)81.63 mmHg.
E)165.93 mmHg.
A)84.64 mmHg.
B)84.30 mmHg.
C)82.96 mmHg.
D)81.63 mmHg.
E)165.93 mmHg.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
39
Calculate the molality of a 20.0% by mass ammonium sulfate (NH4)2SO4 solution. The density of the solution is 1.117 g/mL.
A)0.150 m
B)1.51 m
C)1.70 m
D)1.89 m
E)2.10 m
A)0.150 m
B)1.51 m
C)1.70 m
D)1.89 m
E)2.10 m

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
40
At 10°C one volume of water dissolves 3.10 volumes of chlorine gas at 1.00 atm pressure. What is the Henry's Law constant in mol/L·atm?
A)3.8
B)0.043
C)36.
D)3.1
E)0.13
A)3.8
B)0.043
C)36.
D)3.1
E)0.13

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
41
A solution that contains 55.0 g of ascorbic acid (VitaminC) in 250. g of water freezes at -2.34°C. Calculate the molar mass (in units of g/mol) of the solute. Kf of water is 1.86°C/m.
A) 1.26
B) 10.9
C) 43.6
D) 175
E) 277
A) 1.26
B) 10.9
C) 43.6
D) 175
E) 277

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following aqueous solutions has the highest boiling point? Kb for water is 0.52°C/m.
A)0.2 m KCl
B)0.2 m Na2SO4
C)0.2 m Ca(NO3)2
D)A and B.
E)B and C.
A)0.2 m KCl
B)0.2 m Na2SO4
C)0.2 m Ca(NO3)2
D)A and B.
E)B and C.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
43
When 12.1 g of the sugar sucrose (a nonelectrolyte)are dissolved in exactly 800 g of water, the solution has a freezing point of -0.082°C. What is the molar mass of sucrose? Kf of water is 1.86°C/m.
A)426 g
B)99.2 g
C)178 g
D)266 g
E)343 g
A)426 g
B)99.2 g
C)178 g
D)266 g
E)343 g

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
44
What volume of water should be added to 600. mL of ethanol in order to have a solution that boils at 95.0°C? (For ethanol, Kb = 1.22 °C/m, density = 0.789 g/cm3, boiling point = 78.4°C)
A)186 mL
B)245 mL
C)518 mL
D)116 mL
E)322 mL
A)186 mL
B)245 mL
C)518 mL
D)116 mL
E)322 mL

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following aqueous solutions has the highest osmotic pressure at 25°C?
A)0.2 M KBr
B)0.2 M ethanol
C)0.2 M Na2SO4
D)0.2 M KCl
A)0.2 M KBr
B)0.2 M ethanol
C)0.2 M Na2SO4
D)0.2 M KCl

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
46
What is the osmotic pressure of a 0.25 M solution of sucrose at 37°C? (R = 0.0821 L.atm/K.mol)
A)6.6 * 10-5 atm
B)0.76 atm
C)6.4 atm
D)100 atm
E)940 atm
A)6.6 * 10-5 atm
B)0.76 atm
C)6.4 atm
D)100 atm
E)940 atm

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
47
Pure benzene, C6H6, freezes at 5.5° and boils at 80.1°C. What is the boiling point of a solution consisting of cyclohexane (C6H12)dissolved in benzene if the freezing point of this solution is 0.0°C? (For benzene, Kf = 5.12 °C/m, Kb = 2.53 °C/m; for cyclohexane, Kf = 20.0 °C/m, Kb = 2.79°C/m)
A)82.8°C
B)91.2°C
C)80.9°C
D)77.4°C
E)83.1°C
A)82.8°C
B)91.2°C
C)80.9°C
D)77.4°C
E)83.1°C

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
48
What is the molar mass of toluene if 0.85 g of toluene depresses the freezing point of 100. g of benzene by 0.47°C? Kf of benzene is 5.12°C/m.
A)92.6 g/mol
B)78.0 g/mol
C)10.7 g/mol
D)81.8 g/mol
E)927 g/mol
A)92.6 g/mol
B)78.0 g/mol
C)10.7 g/mol
D)81.8 g/mol
E)927 g/mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
49
What is the osmotic pressure of a solution that contains 13.7 g of propyl alcohol (C3H7OH)dissolved in enough water to make 500. mL of solution at 27°C?
A)0.014 atm
B)0.037 atm
C)0.456 atm
D)0.01 atm
E)11.2 atm
A)0.014 atm
B)0.037 atm
C)0.456 atm
D)0.01 atm
E)11.2 atm

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
50
How many grams of sucrose (C12H22O11, 342.3 g/mol)would be needed to make 2.5 L of a solution with an osmotic pressure of 14 atm at 25°C? (R = 0.0821 L.atm/K.mol)
A)0.57 g
B)6.8 g
C)2.0 * 102 g
D)4.9 * 102 g
E)5.8 * 103 g
A)0.57 g
B)6.8 g
C)2.0 * 102 g
D)4.9 * 102 g
E)5.8 * 103 g

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
51
What mass of ethanol, C2H5OH a nonelectrolyte, must be added to 10.0 L of water to give a solution that freezes at -10.0°C? Assume the density of water is 1.0 g/mL. Kf of water is 1.86°C/m.
A)85.7 kg
B)24.8 kg
C)5.38 kg
D)2.48 kg
E)1.17 kg
A)85.7 kg
B)24.8 kg
C)5.38 kg
D)2.48 kg
E)1.17 kg

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
52
What volume of ethanol (density = 0.7893 g/cm3)should be added to 450. mL of water in order to have a solution that freezes at -15.0°C? (For water, Kf = 1.86 °C/m.)
A)371 mL
B)470 mL
C)212 mL
D)132 mL
E)167 mL
A)371 mL
B)470 mL
C)212 mL
D)132 mL
E)167 mL

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
53
When 24.0 g of glucose (a nonelectrolyte)are dissolved in 500. g of water, the solution has a freezing point of -0.47°C. What is the molar mass of glucose? Kf of water is 1.86°C/m.
A)41.9 g
B)47.5 g
C)54.9 g
D)178 g
E)190. g
A)41.9 g
B)47.5 g
C)54.9 g
D)178 g
E)190. g

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
54
Dissolving a solute such as KOH in a solvent such as water results in
A)an increase in the melting point of the liquid.
B)a decrease in the boiling point of the liquid.
C)a decrease in the vapor pressure of the liquid.
D)no change in the boiling point of the liquid.
A)an increase in the melting point of the liquid.
B)a decrease in the boiling point of the liquid.
C)a decrease in the vapor pressure of the liquid.
D)no change in the boiling point of the liquid.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
55
What is the freezing point of a solution that contains 10.0 g of glucose (C6H12O6)in 100. g of H2O? Kf for water is 1.86°C/m.
A)-0.186°C
B)+0.186°C
C)-0.10°C
D)+0.10°C
E)-1.03°C
A)-0.186°C
B)+0.186°C
C)-0.10°C
D)+0.10°C
E)-1.03°C

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
56
What is the boiling point of an aqueous solution of a nonelectrolyte that has an osmotic pressure of 10.50 atm at 25°C? Kb of water is 0.52°C/m. Assume the density of the solution is the same as that of pure water.
A)0.22°C
B)0.429°C
C)100.43°C
D)99.78°C
E)100.22°C
A)0.22°C
B)0.429°C
C)100.43°C
D)99.78°C
E)100.22°C

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
57
What is the freezing point of an aqueous solution of a nonvolatile solute that has a boiling point of 102.5°C? For water Kf = 1.86°C/m and Kb = 0.52°C/m.
A)-8.94°C
B)-366°C
C)-0.99°C
D)0.99°C
E)8.94°C
A)-8.94°C
B)-366°C
C)-0.99°C
D)0.99°C
E)8.94°C

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
58
What is the freezing point of a solution prepared from 50.0 g ethylene glycol (C2H6O2)and 85.0 g H2O? Kf of water is 1.86°C/m.
A)17.6°C
B)-176°C
C)-1.50°C
D)1.50°C
E)-17.6°C
A)17.6°C
B)-176°C
C)-1.50°C
D)1.50°C
E)-17.6°C

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
59
During osmosis
A)pure solvent diffuses through a membrane but solutes do not.
B)pure solutes diffuse through a membrane but solvent does not.
C)pure solvent and a solution both diffuse at the same time through a membrane.
D)gases diffuse through a membrane into a solution and build up pressure.
A)pure solvent diffuses through a membrane but solutes do not.
B)pure solutes diffuse through a membrane but solvent does not.
C)pure solvent and a solution both diffuse at the same time through a membrane.
D)gases diffuse through a membrane into a solution and build up pressure.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
60
Calculate the freezing point of a solution made from 22.0 g of octane (C8H18)dissolved in 148.0 g of benzene. Benzene freezes at 5.50°C and its Kf value is 5.12°C/m.
A)-1.16°C
B)0.98°C
C)6.66°C
D)12.2°C
E)5.49°C
A)-1.16°C
B)0.98°C
C)6.66°C
D)12.2°C
E)5.49°C

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
61
An aqueous dextrose solution having a density of 1.04 g/cm3 freezes at -1.15°C. Find the osmotic pressure of this solution at 25°C. Kf of water is 1.86 °C/m; molecular mass of dextrose = 180.16 g/mol.
A)13.8 atm
B)14.1 atm
C)15.1 atm
D)12.9 atm
E)120 atm
A)13.8 atm
B)14.1 atm
C)15.1 atm
D)12.9 atm
E)120 atm

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
62
What is the osmotic pressure of a solution prepared from 13.7 g of the electrolyte HCl and enough water to make 0.500 L of solution at 18°C?
A)0.55 atm
B)1.10 atm
C)8.95 atm
D)17.9 atm
E)35.9 atm
A)0.55 atm
B)1.10 atm
C)8.95 atm
D)17.9 atm
E)35.9 atm

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
63
An aqueous fructose solution having a density of 1.049 g/cm3 is found to have an osmotic pressure of 17.0 atm at 25°C. Find the temperature at which this solution freezes. [Given: for water Kf = 1.86 °C/m; molecular mass of fructose = 180.16 g/mol]
A)-1.52°C
B)-1.30°C
C)-1.57°C
D)-1.69°C
E)-1.41°C
A)-1.52°C
B)-1.30°C
C)-1.57°C
D)-1.69°C
E)-1.41°C

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
64
A solution is prepared by adding 40.3 g of Mg(NO3)2 to 127 g of water. Calculate the mole fraction and molality of magnesium nitrate in this solution.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
65
What is the molarity and molality of a solution that is 10.00 % by mass potassium hydrogen carbonate (KHCO3, 100.11 g/mol)and has a density of 1.0650 g/mL?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
66
Arrange the following aqueous solutions in order of increasing boiling points: 0.050 m Mg(NO3)2; 0.100 m ethanol; 0.090 m NaCl.
A)Mg(NO3)2 < NaCl < ethanol
B)ethanol < Mg(NO3)2 < NaCl
C)ethanol < NaCl < Mg(NO3)2
D)NaCl < ethanol < Mg(NO3)2
E)Mg(NO3)2 < ethanol < NaCl
A)Mg(NO3)2 < NaCl < ethanol
B)ethanol < Mg(NO3)2 < NaCl
C)ethanol < NaCl < Mg(NO3)2
D)NaCl < ethanol < Mg(NO3)2
E)Mg(NO3)2 < ethanol < NaCl

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
67
What is the percent by mass of sodium phosphate in a 0.142 M Na3PO4(aq)solution that has a density of 1.015 g/mL?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
68
Calculate the approximate freezing point of a solution made from 21.0 g NaCl and 1.00 * 102 g of H2O. [Kf of water is 1.86°C/m.]
A)3.59°C
B)6.68°C
C)-13.4°C
D)-6.68°C
E)-3.59°C
A)3.59°C
B)6.68°C
C)-13.4°C
D)-6.68°C
E)-3.59°C

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
69
What is the concentration of O2(g)in water at 25°C exposed to a partial pressure of oxygen of 325 mmHg? The Henry's law constant for oxygen gas at 25°C is 1.3 * 10-3 mol/L·atm.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
70
What is the mole fraction of sodium phosphate in a 0.142 M Na3PO4(aq)solution that has a density of 1.015 g/mL?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
71
What is the approximate Na+ ion concentration in a 0.75 M Na2CO3 solution?
A)0.375 M
B)0.75 M
C)1.25 M
D)1.50 M
E)2.25 M
A)0.375 M
B)0.75 M
C)1.25 M
D)1.50 M
E)2.25 M

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
72
A solution is prepared by adding 6.24 g of benzene (C6H6, 78.11 g/mol)to 80.74 g of cyclohexane (C6H12, 84.16 g/mol). Calculate the mole fraction and molality of benzene in this solution.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
73
Consider a 0.90 M Al(NO3)3 solution. This solution has a nitrate ion concentration of
A)0.30 M.
B)0.90 M.
C)0.0 M.
D)8.1 M.
E)2.7 M.
A)0.30 M.
B)0.90 M.
C)0.0 M.
D)8.1 M.
E)2.7 M.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following aqueous solutions has the lowest freezing point?
A)0.18 m KCl
B)0.15 m Na2SO4
C)0.12 m Ca(NO3)2
D)pure water
E)0.20 m C2H6O2 (ethylene glycol)
A)0.18 m KCl
B)0.15 m Na2SO4
C)0.12 m Ca(NO3)2
D)pure water
E)0.20 m C2H6O2 (ethylene glycol)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
75
How many grams of propanol (C3H7OH, 60.10 g/mol)would be needed to make 750 mL of a solution with an osmotic pressure of 25 atm at 25°C? (R = 0.0821 L.atm/K.mol)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following concentration units will not change with temperature: molarity, percent mass, mole fraction, and molality?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
77
What is the molarity of a solution that is 5.50 % by mass oxalic acid (C2H2O4)and has a density of 1.0244 g/mL?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
78
The concentration of nitrogen in water at 25°C was determined to be 7.2 * 10-6 M. Calculate the partial pressure of nitrogen at the surface of the water in mmHg. The Henry's law constant for nitrogen gas at 25°C is 6.8 * 10-4 mol/L·atm?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
79
What is the molality of a 0.142 M Na3PO4(aq)solution that has a density of 1.015 g/mL?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
80
The osmotic pressure of a 0.010 M MgSO4 solution at 25°C is 0.318 atm. Calculate i, the van't Hoff factor, for this MgSO4 solution.
A)0.013
B)1.3
C)1.5
D)2.0
E)76.8
A)0.013
B)1.3
C)1.5
D)2.0
E)76.8

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck


