Deck 20: Transition Elements and Coordination Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/185
Play
Full screen (f)
Deck 20: Transition Elements and Coordination Chemistry
1
Which of the following species has the ground-state electron configuration [Ar]3d4?
A)Ti
B)V2+
C)Cr2+
D)Fe2+
A)Ti
B)V2+
C)Cr2+
D)Fe2+
Cr2+
2
Which transition metal has the anomalous ground-state electron configuration: [Kr] 4d10?
A)Rh
B)Pd
C)Ag
D)Cd
A)Rh
B)Pd
C)Ag
D)Cd
Pd
3
What is the ground-state electron configuration for the element chromium (Z = 24)?
A)[Ne] 4s2 3d4
B)[Ar] 4s2 3d4
C)[Ar] 4s1 3d5
D)[Ar] 3d6
A)[Ne] 4s2 3d4
B)[Ar] 4s2 3d4
C)[Ar] 4s1 3d5
D)[Ar] 3d6
[Ar] 4s1 3d5
4
What is the ground-state electron configuration for Co2+ (Z = 27)?
A)[Ar] 4s2 3d9
B)[Ar] 4s2 3d5
C)[Ar] 3d7
D)[Ar] 4s1 3d6
A)[Ar] 4s2 3d9
B)[Ar] 4s2 3d5
C)[Ar] 3d7
D)[Ar] 4s1 3d6

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
5
What is the d orbital-filling diagram for Fe3+ (Z = 26)? 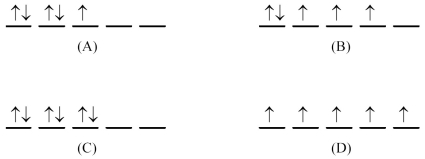
A)(A)
B)(B)
C)(C)
D)(D)

A)(A)
B)(B)
C)(C)
D)(D)

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
6
Which first row transition element has the highest melting point?
A)Sc
B)V
C)Fe
D)Zn
A)Sc
B)V
C)Fe
D)Zn

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following elements is an inner transition metal?
A)Eu
B)Fe
C)Ru
D)Sc
A)Eu
B)Fe
C)Ru
D)Sc

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
8
Which element is most likely to have an anomalous electron configuration?
A)Y
B)Tc
C)Ag
D)Cd
A)Y
B)Tc
C)Ag
D)Cd

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
9
What is the characteristic outer electron configuration for transition elements?
A)(n - 1)d10-xns2
B)(n)d10-xns2
C)(n + 1)d10-xns1
D)(n - 1)d10-x(n + 1)s2
A)(n - 1)d10-xns2
B)(n)d10-xns2
C)(n + 1)d10-xns1
D)(n - 1)d10-x(n + 1)s2

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
10
How many d electrons are there in MnO4-?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
11
What is the ground-state electron configuration for the element cobalt (Z = 27)?
A)[Ar] 4s2 3d7
B)[Ar] 3d9
C)[Ar] 4s2 4p6 5s1
D)[Ar] 3d7
A)[Ar] 4s2 3d7
B)[Ar] 3d9
C)[Ar] 4s2 4p6 5s1
D)[Ar] 3d7

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
12
In which blocks of the periodic table are the transition series and inner transition series elements found?
A)d,p
B)d,f
C)s,d
D)s,p
A)d,p
B)d,f
C)s,d
D)s,p

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
13
What is the ground-state electron configuration for Cr in Cr2O72-?
A)[Ar] 4s1 3d5
B)[Ar] 4s2 3d6
C)[Ar] 3d4
D)[Ar] 3d0
A)[Ar] 4s1 3d5
B)[Ar] 4s2 3d6
C)[Ar] 3d4
D)[Ar] 3d0

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
14
Element M has the valence electron configuration 3d6 4s2.What is the valence electron configuration of The M3+ ion?
A)3d5
B)3d3 4s2
C)3d4 4s1
D)3d9 4s2
A)3d5
B)3d3 4s2
C)3d4 4s1
D)3d9 4s2

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
15
Which is a third transition series element?
A)Al
B)Mg
C)Pr
D)Pt
A)Al
B)Mg
C)Pr
D)Pt

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
16
The number of transition series is
A)one
B)two
C)four
D)seven
A)one
B)two
C)four
D)seven

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
17
How many valence electrons does the element Co have?
A)2
B)7
C)8
D)9
A)2
B)7
C)8
D)9

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
18
Which transition element has the highest melting point?
A)Fe
B)V
C)Mo
D)W
A)Fe
B)V
C)Mo
D)W

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
19
Transition series elements are all
A)gases.
B)metals.
C)nonmetals.
D)semimetals.
A)gases.
B)metals.
C)nonmetals.
D)semimetals.

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
20
What two transition elements have the highest electrical conductivity of any elements at room temperature?
A)copper and iron
B)copper and silver
C)iron and chromium
D)silver and gold
A)copper and iron
B)copper and silver
C)iron and chromium
D)silver and gold

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
21
Which is the strongest oxidizing agent under acidic conditions?
A)Cr2+
B)Cr3+
C)CrO42-
D)Cr2O72-
A)Cr2+
B)Cr3+
C)CrO42-
D)Cr2O72-

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following transition elements (Sc,Ti,V,Mn,and Cu)have positive oxidation potentials?
A)Sc
B)Cu
C)Sc,Ti,and V
D)Sc,Ti,V,and Mn
A)Sc
B)Cu
C)Sc,Ti,and V
D)Sc,Ti,V,and Mn

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following chromium species is the strongest acid?
A)Cr(OH)2
B)Cr(OH)3
C)CrO2(OH)2
D)CrO42-
A)Cr(OH)2
B)Cr(OH)3
C)CrO2(OH)2
D)CrO42-

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
24
What oxidation state(s)is(are)exhibited by all first row transition elements except scandium?
A)+2
B)+3
C)+2 and +3
D)+2,+3 and +4
A)+2
B)+3
C)+2 and +3
D)+2,+3 and +4

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
25
Which transition element is difficult to oxidize with hydronium ion?
A)Cr
B)Cu
C)Mn
D)Ti
A)Cr
B)Cu
C)Mn
D)Ti

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
26
What are two of the major components of stainless steel?
A)iron and carbon
B)iron and chromium
C)iron and titanium
D)iron and tungsten
A)iron and carbon
B)iron and chromium
C)iron and titanium
D)iron and tungsten

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
27
What is the highest possible oxidation state for chromium?
A)+3
B)+4
C)+5
D)+6
A)+3
B)+4
C)+5
D)+6

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
28
Which chromium species exists only under acidic conditions?
A)Cr(OH)2
B)Cr(OH)4-
C)CrO42-
D)Cr2O72-
A)Cr(OH)2
B)Cr(OH)4-
C)CrO42-
D)Cr2O72-

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
29
What chemical equation represents the best method for obtaining pure chromium?
A)FeCr2O4(s)+ 4 C(s)+ heat → Fe(s)+ 2 Cr(s)+ 4 CO(g)
B)Cr2O3(s)+ 2 Al(s)+ heat → 2 Cr(s)+ Al2O3(s)
C)Cr2O3(s)+ 2 Fe(s)+ heat → Fe2O3(s)+ 2 Cr(s)
D)Cr2+(aq)+ H2(g)→ Cr(s)+ 2 H+(aq)
A)FeCr2O4(s)+ 4 C(s)+ heat → Fe(s)+ 2 Cr(s)+ 4 CO(g)
B)Cr2O3(s)+ 2 Al(s)+ heat → 2 Cr(s)+ Al2O3(s)
C)Cr2O3(s)+ 2 Fe(s)+ heat → Fe2O3(s)+ 2 Cr(s)
D)Cr2+(aq)+ H2(g)→ Cr(s)+ 2 H+(aq)

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
30
What statement is most inconsistent about the chemistry of iron?
A)Iron(III)hydroxide is very soluble and reacts readily with hydroxide to form Fe(OH)4-.
B)Iron reacts with hydrochloric acid in the absence of air to yield iron(II)ion and hydrogen gas.
C)Iron reacts with nitric acid to yield iron(III)ion and nitric oxide.
D)The most common oxidation states of iron are +2 (ferrous)and +3 (ferric).
A)Iron(III)hydroxide is very soluble and reacts readily with hydroxide to form Fe(OH)4-.
B)Iron reacts with hydrochloric acid in the absence of air to yield iron(II)ion and hydrogen gas.
C)Iron reacts with nitric acid to yield iron(III)ion and nitric oxide.
D)The most common oxidation states of iron are +2 (ferrous)and +3 (ferric).

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
31
In the two half-reactions shown below,which chromium species is the strongest oxidizing agent?
Cr2O72-(aq)+ 14 H+(aq)+ 6 e- → 2 Cr3+(aq)+ 7 H2O(l)E° = + 1.33 V
CrO42-(aq)+ 4 H2O(l)+ 6 e- → Cr(OH)3(s)+ 5 OH-(aq)E° = - 0.13 V
A)Cr2O72-
B)Cr3+
C)CrO42-
D)Cr(OH)3
Cr2O72-(aq)+ 14 H+(aq)+ 6 e- → 2 Cr3+(aq)+ 7 H2O(l)E° = + 1.33 V
CrO42-(aq)+ 4 H2O(l)+ 6 e- → Cr(OH)3(s)+ 5 OH-(aq)E° = - 0.13 V
A)Cr2O72-
B)Cr3+
C)CrO42-
D)Cr(OH)3

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
32
What is the strongest oxidizing agent of the following set: MnCl2,Mn(OH)3,MnO2,KMnO4?
A)MnCl2
B)Mn(OH)3
C)MnO2
D)KMnO4
A)MnCl2
B)Mn(OH)3
C)MnO2
D)KMnO4

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
33
What statement is inconsistent with the chemistry of iron?
A)Iron is the fourth most abundant element in the earth's crust.
B)Iron is obtained from the reduction of hematite (Fe2O3)and magnetite (Fe3O4)by carbon in a blast furnace.
C)Iron is a relatively hard metal and it is relatively unreactive with haloacids.
D)The majority of iron in a healthy human is present in the oxygen-carrying protein hemoglobin.
A)Iron is the fourth most abundant element in the earth's crust.
B)Iron is obtained from the reduction of hematite (Fe2O3)and magnetite (Fe3O4)by carbon in a blast furnace.
C)Iron is a relatively hard metal and it is relatively unreactive with haloacids.
D)The majority of iron in a healthy human is present in the oxygen-carrying protein hemoglobin.

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
34
What statement is most inconsistent with the chemistry of transition elements?
A)Bromide,chloride and iodide stabilize the higher oxidation states of the transition elements.
B)Early transition metal ions with the metal in its lowest oxidation state are good reducing agents.
C)Ions that have transition metal in their highest oxidation state tend to be good oxidizing agents.
D)The stability of the higher oxidation states increases down a periodic group.
A)Bromide,chloride and iodide stabilize the higher oxidation states of the transition elements.
B)Early transition metal ions with the metal in its lowest oxidation state are good reducing agents.
C)Ions that have transition metal in their highest oxidation state tend to be good oxidizing agents.
D)The stability of the higher oxidation states increases down a periodic group.

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
35
Though we would expect an increase in atomic radii going down a group from the second to the third transition series of elements,the actual radii are nearly identical.The term commonly used to describe this phenomenon is the ________.
A)atomic disparity
B)effective nuclear charge
C)lanthanide contraction
D)transition default
A)atomic disparity
B)effective nuclear charge
C)lanthanide contraction
D)transition default

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
36
What is the strongest oxidizing agent of the following set: VCl2,CrCl3,KMnO4,KReO4?
A)VCl2
B)CrCl3
C)KMnO4
D)KReO4
A)VCl2
B)CrCl3
C)KMnO4
D)KReO4

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following elements has the highest density?
A)Cr
B)Ni
C)Os
D)Ta
A)Cr
B)Ni
C)Os
D)Ta

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
38
For transition elements,which of the following occurs as the effective nuclear charge increases?
A)The atomic radius increases.
B)The density increases.
C)Both the atomic radius and the density increase.
D)The atomic radius decreases and the density increases.
A)The atomic radius increases.
B)The density increases.
C)Both the atomic radius and the density increase.
D)The atomic radius decreases and the density increases.

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
39
Vanadium in the +5 oxidation is most often found as compounds of ________.
A)bromides
B)chlorides
C)fluorides
D)iodides
A)bromides
B)chlorides
C)fluorides
D)iodides

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
40
Which is not a characteristic reaction of chromium metal or chromium(II)ion?
A)Cr(s)+ 2 H+(aq)→ Cr2+(aq)+ H2(g)
B)4 Cr2+(aq)+ O2(g)+ 4 H+(aq)→ 4 Cr3+(aq)+ 2 H2O(l)
C)Cr(OH)2(s)+ 2 H3O+(aq)→ Cr2+(aq)+ 4 H2O(l)
D)Cr(OH)2(s)+ OH-(aq)→ Cr(OH)3-(aq)
A)Cr(s)+ 2 H+(aq)→ Cr2+(aq)+ H2(g)
B)4 Cr2+(aq)+ O2(g)+ 4 H+(aq)→ 4 Cr3+(aq)+ 2 H2O(l)
C)Cr(OH)2(s)+ 2 H3O+(aq)→ Cr2+(aq)+ 4 H2O(l)
D)Cr(OH)2(s)+ OH-(aq)→ Cr(OH)3-(aq)

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
41
Describe what happens when 3.0 M NH3 is slowly added to an aqueous solution of CuSO4.
A)A blue precipitate of Cu(OH)2 forms.
B)A royal blue complex of [Cu(NH3)4]2+ is formed.
C)A blue precipitate of Cu(OH)2 is formed which is then converted to the royal blue complex [Cu(NH3)4]2+.
D)A royal blue complex of [Cu(NH3)4]2+ is formed which then is converted to a blue precipitate of Cu(OH)2.
A)A blue precipitate of Cu(OH)2 forms.
B)A royal blue complex of [Cu(NH3)4]2+ is formed.
C)A blue precipitate of Cu(OH)2 is formed which is then converted to the royal blue complex [Cu(NH3)4]2+.
D)A royal blue complex of [Cu(NH3)4]2+ is formed which then is converted to a blue precipitate of Cu(OH)2.

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following can function as a bidentate ligand?
A)CO
B)OH-
C)NH3
D)C2O42-
A)CO
B)OH-
C)NH3
D)C2O42-

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
43
Ethylenediaminetetraacetate ion (EDTA4-)is commonly referred to as a ________ ligand.
A)monodentate
B)bidentate
C)tetradentate
D)hexadentate
A)monodentate
B)bidentate
C)tetradentate
D)hexadentate

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
44
What is the coordination number of the Fe atom in K3[Fe(C2O4)3]?
A)2
B)3
C)4
D)6
A)2
B)3
C)4
D)6

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following can function as a chelating agent?
A)CN-
B)CO
C)H2NCH2CH2NH2
D)NCS-
A)CN-
B)CO
C)H2NCH2CH2NH2
D)NCS-

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
46
What is the compound responsible for the green patina seen on bronze monuments?
A)CuCO3
B)Cu2(OH)2CO3
C)Cu(OH)2
D)Cu2(OH)2SO4
A)CuCO3
B)Cu2(OH)2CO3
C)Cu(OH)2
D)Cu2(OH)2SO4

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
47
A chromium(III)ion forms a complex ion with two ammonia molecules and four thiocyanate ions.What is the formula of the complex ion?
A)[Cr(NH3)2(NCS)4]3+
B)[Cr(NH4)2(NCS)4]+
C)[Cr(NH3)2(NCS)4]-
D)[Cr(NH3)2(NCS)4]4-
A)[Cr(NH3)2(NCS)4]3+
B)[Cr(NH4)2(NCS)4]+
C)[Cr(NH3)2(NCS)4]-
D)[Cr(NH3)2(NCS)4]4-

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
48
Which one of the following compounds is not consistent with the terminology of a complex?
A)[Cu(NH3)4]2+
B)CuSO4 ∙ 5H2O
C)K3[Fe(CN)6]
D)[Cr(NH3)6]Cl3
A)[Cu(NH3)4]2+
B)CuSO4 ∙ 5H2O
C)K3[Fe(CN)6]
D)[Cr(NH3)6]Cl3

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is a disproportionation reaction?
A)Cu2S(l)+ O2(g)→ 2 Cu(l)+ SO2(g)
B)3 Cu(s)+ 2 NO3-(aq)+ 8 H+(aq)→ 3 Cu2+(aq)+ 2 NO(g)+ 4 H2O(l)
C)2 Cu+(aq)→ Cu(s)+ Cu2+(aq)
D)Cu2+(aq)+ 4 NH3(aq)→ Cu(NH3)42+(aq)
A)Cu2S(l)+ O2(g)→ 2 Cu(l)+ SO2(g)
B)3 Cu(s)+ 2 NO3-(aq)+ 8 H+(aq)→ 3 Cu2+(aq)+ 2 NO(g)+ 4 H2O(l)
C)2 Cu+(aq)→ Cu(s)+ Cu2+(aq)
D)Cu2+(aq)+ 4 NH3(aq)→ Cu(NH3)42+(aq)

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
50
What statement is inconsistent with the chemistry of copper?
A)It has a high electrical conductivity and is widely used to make electrical wiring.
B)It is commonly found in the elemental state.
C)It is a reddish colored metal that accounts for only 0.0068% of the earth's crust by mass.
D)It is used to make corrosion-resistant water pipes because it has a positive oxidation potential.
A)It has a high electrical conductivity and is widely used to make electrical wiring.
B)It is commonly found in the elemental state.
C)It is a reddish colored metal that accounts for only 0.0068% of the earth's crust by mass.
D)It is used to make corrosion-resistant water pipes because it has a positive oxidation potential.

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
51
Write the chemical formula for aquabromobis(ethylenediamine)chromium(III)chloride.
A)[CrBr(H2O)(en)]Cl
B)[CrBr2(H2O)(en)]Cl2
C)[CrBr(H2O)(en)2]Cl2
D)[CrBr(H2O)(en)2]Cl3
A)[CrBr(H2O)(en)]Cl
B)[CrBr2(H2O)(en)]Cl2
C)[CrBr(H2O)(en)2]Cl2
D)[CrBr(H2O)(en)2]Cl3

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following can function as a bidentate ligand?
A)CN-
B)NCS-
C)H2NCH2CO2-
D)All of these can function as bidentate ligands.
A)CN-
B)NCS-
C)H2NCH2CO2-
D)All of these can function as bidentate ligands.

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
53
What is the strongest oxidizing agent of the following set: FeO,Fe2O3,Fe3O4,FeO42-?
A)FeO
B)Fe2O3
C)Fe3O4
D)FeO42-
A)FeO
B)Fe2O3
C)Fe3O4
D)FeO42-

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
54
What is the oxidation state of the Cr atom in [Ni(en)3]3[Cr(CN)6]2?
A)+2
B)+3
C)+4
D)+6
A)+2
B)+3
C)+4
D)+6

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
55
What is the coordination number of the Au atom in K [Au(CN)2(SCN)2]?
A)2
B)3
C)4
D)6
A)2
B)3
C)4
D)6

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
56
Which is a complex ion?
A)CrCl3
B)CrO42-
C)[Cr(H2O)6]3+
D)Cr2O72-
A)CrCl3
B)CrO42-
C)[Cr(H2O)6]3+
D)Cr2O72-

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
57
Using the following reduction potentials for copper determine the unstable copper compound.
Cu+(aq)+ e- → Cu(s)E° = +0.52 V
Cu2+(aq)+ e- → Cu+(aq)E° = +0.15 V
A)CuCl
B)CuCl2
C)CuSO4
D)Cu(OH)2
Cu+(aq)+ e- → Cu(s)E° = +0.52 V
Cu2+(aq)+ e- → Cu+(aq)E° = +0.15 V
A)CuCl
B)CuCl2
C)CuSO4
D)Cu(OH)2

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
58
Which ligand when bonded to a metal would be incorrectly named?
A)H2O,aqua
B)NH3,ammonia
C)CO,carbonyl
D)F-,fluoro
A)H2O,aqua
B)NH3,ammonia
C)CO,carbonyl
D)F-,fluoro

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
59
When the oxalate ion,C2O42- is bonded to the iron(III)ion in the complex ion [Fe(C2O4)3]3-,a ________-membered chelate ring is formed.
A)3
B)4
C)5
D)6
A)3
B)4
C)5
D)6

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
60
What is the oxidation state of the Co atom in [Co(NH3)5Cl](NO3)2?
A)+2
B)+3
C)+4
D)+6
A)+2
B)+3
C)+4
D)+6

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
61
What is the correct formula for tetraamminecarbonatoiron(III)chloride?
A)(NH3)4[FeCO3]Cl
B)[Fe(CO3)(NH3)4]Cl
C)[Fe(CO3)(NH3)4]Cl2
D)[Fe(CO3)Cl(NH3)4]
A)(NH3)4[FeCO3]Cl
B)[Fe(CO3)(NH3)4]Cl
C)[Fe(CO3)(NH3)4]Cl2
D)[Fe(CO3)Cl(NH3)4]

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
62
Which complex cannot exist as enantiomers?
A)[CoCl2(H2O)4]+
B)[Co(en)3]3+
C)cis-[CrCl2(C2O4)2]+
D)[Fe(C2O4)2(en)]-
A)[CoCl2(H2O)4]+
B)[Co(en)3]3+
C)cis-[CrCl2(C2O4)2]+
D)[Fe(C2O4)2(en)]-

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
63
Which one of the following objects is chiral?
A)a bottle
B)a chair
C)a glass
D)a glove
A)a bottle
B)a chair
C)a glass
D)a glove

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
64
Write the chemical formula for pentaamminenitritocobalt(III)ion.
A)[Co(NO)(NH3)5]3+
B)[Co(NO2)(NH3)5]2+
C)[Co(ONO)(NH3)5]2+
D)[Co(NH3)5(N2O)]2+
A)[Co(NO)(NH3)5]3+
B)[Co(NO2)(NH3)5]2+
C)[Co(ONO)(NH3)5]2+
D)[Co(NH3)5(N2O)]2+

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
65
What is the name of the complex [Ni(en)3]3[Cr(CN)6]2?
A)ethylenediaminenickel(III)hexacyanochromate(II)
B)tris(ethylenediamine)nickel(III)hexacyanochromate(II)
C)tris(ethylenediamine)nickel(II)hexacyanochromate(III)
D)bis(ethylenediamine)nickel(II)hexacyanochromate(III)
A)ethylenediaminenickel(III)hexacyanochromate(II)
B)tris(ethylenediamine)nickel(III)hexacyanochromate(II)
C)tris(ethylenediamine)nickel(II)hexacyanochromate(III)
D)bis(ethylenediamine)nickel(II)hexacyanochromate(III)

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
66
Which complex is optically active?
A)[CoCl4en]2-
B)trans-[CrCl2(en)2]+
C)cis-[CrCl2(en)2]+
D)[PtCl2(NH3)2]
A)[CoCl4en]2-
B)trans-[CrCl2(en)2]+
C)cis-[CrCl2(en)2]+
D)[PtCl2(NH3)2]

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
67
A complex ion that has a broad absorption band at 625 nm in its visible absorption spectrum will appear to be
A)blue
B)colorless
C)red
D)yellow
A)blue
B)colorless
C)red
D)yellow

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
68
The compounds [Cr(H2O)6]Cl3 and [CrCl3(H2O)3] ∙ 3H2O are examples of ________.
A)diastereoisomers
B)enantiomers
C)ionization isomers
D)linkage isomers
A)diastereoisomers
B)enantiomers
C)ionization isomers
D)linkage isomers

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
69
What is the name of the complex [Ni(H2O)4(NH2CH2CH2NH2)]SO4 ∙ 5H2O?
A)aquaethylenediaminenickel(II)sulfate hydrate
B)tetraaquaethylenediaminenickel(II)sulfate pentahydrate
C)tetraaquabis(ethylenediamine)nickel(II)sulfate pentahydrate
D)tetraaquabis(ethylenediamine)nickel(III)sulfate pentahydrate
A)aquaethylenediaminenickel(II)sulfate hydrate
B)tetraaquaethylenediaminenickel(II)sulfate pentahydrate
C)tetraaquabis(ethylenediamine)nickel(II)sulfate pentahydrate
D)tetraaquabis(ethylenediamine)nickel(III)sulfate pentahydrate

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
70
What is the name of the complex ion [AuBrCl(CN)2]-?
A)bromochlorodicyanogold(I)ion
B)bromochlorodicyanoaurate(III)ion
C)bromochlorodicyanoargentate(III)ion
D)bromochlorodicyanoaurate(IV)ion
A)bromochlorodicyanogold(I)ion
B)bromochlorodicyanoaurate(III)ion
C)bromochlorodicyanoargentate(III)ion
D)bromochlorodicyanoaurate(IV)ion

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
71
Which ion has cis and trans isomers?
A)[PdCl3NH3]-
B)[Pt(CN)5NH3]-
C)[PtCl2(CN)2]2-
D)[Pt(C2O4)2]2-
A)[PdCl3NH3]-
B)[Pt(CN)5NH3]-
C)[PtCl2(CN)2]2-
D)[Pt(C2O4)2]2-

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
72
In order to form a neutral compound,hexafluoroaluminate would require
A)three K+ ions.
B)three Cl- ions.
C)six Na+ ions.
D)six Br- ions.
A)three K+ ions.
B)three Cl- ions.
C)six Na+ ions.
D)six Br- ions.

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
73
Which definition best describes isomers that are non-superimposable mirror images of each other that rotate plane polarized light to the same degree but in opposite directions?
A)diastereoisomers
B)enantiomers
C)linkage isomers
D)racemic mixture
A)diastereoisomers
B)enantiomers
C)linkage isomers
D)racemic mixture

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
74
Which pair of isomers illustrates the concept of ionization isomers?
A)[Cr(SCN)(NH3)5]2+ and [Cr(NCS)(NH3)5]2+
B)[CoCl(NH3)5]SO4 and [Co(SO4)(NH3)5]Cl
C)cis -[PtCl2(NH3)2] and trans -[PtCl2(NH3)2]
D)(+)-[Co(en)3]3+ and (-)-[Co(en)3]3+
A)[Cr(SCN)(NH3)5]2+ and [Cr(NCS)(NH3)5]2+
B)[CoCl(NH3)5]SO4 and [Co(SO4)(NH3)5]Cl
C)cis -[PtCl2(NH3)2] and trans -[PtCl2(NH3)2]
D)(+)-[Co(en)3]3+ and (-)-[Co(en)3]3+

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
75
What hybridization scheme is used for Ni in the square planar complex of [Ni(CN)4]2-?
A)sp3
B)dsp2
C)dsp3
D)d2sp3
A)sp3
B)dsp2
C)dsp3
D)d2sp3

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
76
How many unpaired electrons are present in the high spin form of the [CoF6]3- complex and what metal orbitals are used in bonding?
A)0 unpaired electrons and 4s,4p and 4d orbitals to give sp3d2
B)4 unpaired electrons and 4s,4p and 4d orbitals to give sp3d2
C)0 unpaired electrons and 3d,4s,and 4p orbitals to give d2sp3
D)4 unpaired electrons and 3d,4s,and 4p orbitals to give d2sp3
A)0 unpaired electrons and 4s,4p and 4d orbitals to give sp3d2
B)4 unpaired electrons and 4s,4p and 4d orbitals to give sp3d2
C)0 unpaired electrons and 3d,4s,and 4p orbitals to give d2sp3
D)4 unpaired electrons and 3d,4s,and 4p orbitals to give d2sp3

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
77
Identify the classification of isomers illustrated by [Co(NO2)(NH3)5]2+ and [Co(ONO)(NH3)5]2+.
A)linkage isomers
B)ionization isomers
C)geometric isomers
D)optical isomers
A)linkage isomers
B)ionization isomers
C)geometric isomers
D)optical isomers

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
78
What type of hybrid orbitals are used by the Ti atom to form chemical bonds in the complex ion [Ti(H2O)6]3+?
A)sp3
B)dsp2
C)dsp3
D)d2sp3
A)sp3
B)dsp2
C)dsp3
D)d2sp3

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
79
Which metal ion is most likely to form a square planar complex ion with CN-?
A)Co2+
B)Cu2+
C)Ni2+
D)Zn2+
A)Co2+
B)Cu2+
C)Ni2+
D)Zn2+

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck
80
The complex cis-[CoCl(NH3)(NH2CH2CH2NH2)2]2+ was resolved into optical isomers in 1911 by Alfred Werner,demonstrating the octahedral geometry of the ion.Name this complex ion.
A)cis-chloroammineethylenediaminecobalt(II)ion
B)cis-amminechloroethylenediaminecobalt(III)ion
C)cis-amminechlorobis(ethylenediamine)cobalt(II)ion
D)cis-amminechlorobis(ethylenediamine)cobalt(III)ion
A)cis-chloroammineethylenediaminecobalt(II)ion
B)cis-amminechloroethylenediaminecobalt(III)ion
C)cis-amminechlorobis(ethylenediamine)cobalt(II)ion
D)cis-amminechlorobis(ethylenediamine)cobalt(III)ion

Unlock Deck
Unlock for access to all 185 flashcards in this deck.
Unlock Deck
k this deck


