Exam 20: Transition Elements and Coordination Chemistry
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
The coordination number of platinum in Pt(NH3)2Cl2 is ________.
Free
(Short Answer)
4.8/5  (41)
(41)
Correct Answer:
4
Which of the following can function as a bidentate ligand?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
C
Transition elements are located in the ________-block of the periodic table.
Free
(Short Answer)
4.8/5  (34)
(34)
Correct Answer:
D
The formula for tetraamminedichlorochromium(III)chloride is ________.
(Short Answer)
4.9/5  (33)
(33)
The element that forms a 2+ ion with the electron configuration [Xe] 4f14 5d8 is ________.
(Short Answer)
4.8/5  (38)
(38)
Inner transition elements are found in the ________-block of the periodic table.
(Short Answer)
4.8/5  (41)
(41)
What is the compound responsible for the green patina seen on bronze monuments?
(Multiple Choice)
4.8/5  (28)
(28)
The first transition series metals are shown below. 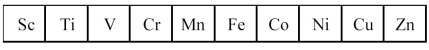 -Which has the greatest density?
-Which has the greatest density?
(Multiple Choice)
4.8/5  (38)
(38)
What hybridization scheme is used for Ni in the square planar complex of [Ni(CN)4]2-?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following chromium species is the strongest acid?
(Multiple Choice)
4.7/5  (42)
(42)
Which is the strongest oxidizing agent under acidic conditions?
(Multiple Choice)
4.8/5  (24)
(24)
What is the strongest oxidizing agent of the following set: VCl2,CrCl3,KMnO4,KReO4?
(Multiple Choice)
4.8/5  (42)
(42)
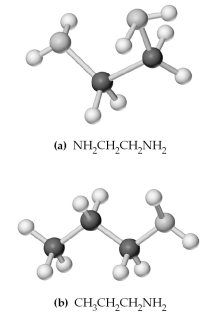
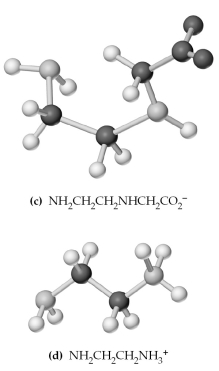 -Which of the above are bidentate or tridentate ligands,capable of forming chelate rings?
-Which of the above are bidentate or tridentate ligands,capable of forming chelate rings?
(Multiple Choice)
4.9/5  (43)
(43)
What two transition elements have the highest electrical conductivity of any elements at room temperature?
(Multiple Choice)
4.8/5  (34)
(34)
![-Which element indicated on the above periodic table has the electron configuration [Ar] 3d<sup>3</sup> 4s<sup>2</sup>?](https://storage.examlex.com/TB4939/11ea7a38_c9a7_8e4f_aa4c_ff5f6fa7120c_TB4939_00_TB4939_00_TB4939_00_TB4939_00_TB4939_00_TB4939_00.jpg) -Which element indicated on the above periodic table has the electron configuration [Ar] 3d3 4s2?
-Which element indicated on the above periodic table has the electron configuration [Ar] 3d3 4s2?
(Multiple Choice)
4.8/5  (37)
(37)
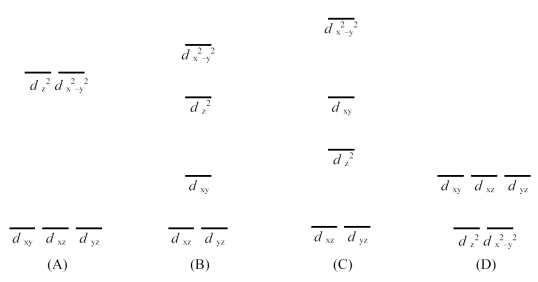 -Which is the crystal field energy level diagram for a square planar ML4 complex that contains no ligands on the z-axis?
-Which is the crystal field energy level diagram for a square planar ML4 complex that contains no ligands on the z-axis?
(Multiple Choice)
4.9/5  (39)
(39)
Showing 1 - 20 of 185
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)