Deck 2: Atoms, molecules, and Ions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/257
Play
Full screen (f)
Deck 2: Atoms, molecules, and Ions
1
The observation that hydrogen and oxygen can react to form two compounds with different chemical and physical properties,one having an O:H mass ratio = 8:1 and the other having an O:H mass ratio = 16:1 is consistent with the law of
A)definite proportions.
B)energy conservation.
C)mass conservation.
D)multiple proportions.
A)definite proportions.
B)energy conservation.
C)mass conservation.
D)multiple proportions.
multiple proportions.
2
The observation that 4.0 g of hydrogen reacts with 32.0 g of oxygen to form a product with
O:H mass ratio = 8:1,and 6.0 g of hydrogen reacts with 48.0 g of oxygen to form the same product
With O/H mass ratio = 8:1 is evidence for the law of
A)definite proportions.
B)energy conservation.
C)mass conservation.
D)multiple proportions.
O:H mass ratio = 8:1,and 6.0 g of hydrogen reacts with 48.0 g of oxygen to form the same product
With O/H mass ratio = 8:1 is evidence for the law of
A)definite proportions.
B)energy conservation.
C)mass conservation.
D)multiple proportions.
definite proportions.
3
The existence of electrons in atoms of all elements was demonstrated by
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these
Thomson's cathode ray tube experiment.
4
Which of the following is a part of Dalton's atomic theory?
A)Atoms are rearranged but not changed during a chemical reaction.
B)Atoms break down during radioactive decay.
C)Atoms contain protons,neutrons,and electrons.
D)Isotopes of the same element have different masses.
A)Atoms are rearranged but not changed during a chemical reaction.
B)Atoms break down during radioactive decay.
C)Atoms contain protons,neutrons,and electrons.
D)Isotopes of the same element have different masses.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
5
According to history,the concept that all matter is composed of atoms was first proposed by
A)the Greek philosopher Democritus,but not widely accepted until modern times.
B)Dalton,but not widely accepted until the work of Mendeleev.
C)Dalton,but not widely accepted until the work of Einstein.
D)Dalton,and widely accepted within a few decades.
A)the Greek philosopher Democritus,but not widely accepted until modern times.
B)Dalton,but not widely accepted until the work of Mendeleev.
C)Dalton,but not widely accepted until the work of Einstein.
D)Dalton,and widely accepted within a few decades.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
6
A proton is approximately
A)200 times larger than an electron.
B)2000 times larger than an electron.
C)200 times smaller than an electron.
D)2000 times smaller than an electron.
A)200 times larger than an electron.
B)2000 times larger than an electron.
C)200 times smaller than an electron.
D)2000 times smaller than an electron.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
7
The mass number of an atom is equal to the number of
A)electrons.
B)neutrons.
C)protons.
D)protons plus neutrons.
A)electrons.
B)neutrons.
C)protons.
D)protons plus neutrons.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
8
A sample of pure lithium carbonate contains 18.8% lithium by mass.What is the % lithium by mass in a sample of pure lithium carbonate that has twice the mass of the first sample?
A)9)40%
B)18.8%
C)37.6%
D)75.2%
A)9)40%
B)18.8%
C)37.6%
D)75.2%

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
9
The existence of neutrons in the nucleus of an atom was demonstrated by
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
10
Methane and oxygen react to form carbon dioxide and water.What mass of water is formed if 3.2 g of methane reacts with 12.8 g of oxygen to produce 8.8 g of carbon dioxide?
A)7)2 g
B)8)8 g
C)14.8 g
D)16.0 g
A)7)2 g
B)8)8 g
C)14.8 g
D)16.0 g

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
11
Sodium metal and water react to form hydrogen and sodium hydroxide.If 5.98 g of sodium react with water to form 0.26 g of hydrogen and 10.40 g of sodium hydroxide,what mass of water was consumed in the reaction?
A)4)68 g
B)5)98 g
C)10.14 g
D)10.66 g
A)4)68 g
B)5)98 g
C)10.14 g
D)10.66 g

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
12
Most of the alpha particles directed at a thin gold foil in Rutherford's experiment
A)bounced directly back from the foil.
B)passed directly through the foil undeflected.
C)passed through the foil but were deflected at an angle.
D)were absorbed by the foil.
A)bounced directly back from the foil.
B)passed directly through the foil undeflected.
C)passed through the foil but were deflected at an angle.
D)were absorbed by the foil.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements is not a postulate of Dalton's atomic theory?
A)Each element is characterized by the mass of its atoms.
B)Atoms are composed of protons,neutrons,and electrons.
C)Chemical reactions only rearrange atomic combinations.
D)Elements are composed of atoms.
A)Each element is characterized by the mass of its atoms.
B)Atoms are composed of protons,neutrons,and electrons.
C)Chemical reactions only rearrange atomic combinations.
D)Elements are composed of atoms.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
14
The charge-to-mass ratio of an electron was established by
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
15
The current model of the atom in which essentially all of an atom's mass is contained in a very small nucleus,whereas most of an atom's volume is due to the space in which the atom's electrons move was established by
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is not explained by Dalton's atomic theory?
A)conservation of mass during a chemical reaction
B)the existence of more than one isotope of an element
C)the law of definite proportions
D)the law of multiple proportions
A)conservation of mass during a chemical reaction
B)the existence of more than one isotope of an element
C)the law of definite proportions
D)the law of multiple proportions

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
17
The symbol that is usually used to represent atomic number is ________.
A)A
B)N
C)X
D)Z
A)A
B)N
C)X
D)Z

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
18
Which subatomic particle has the smallest mass?
A)a proton
B)a neutron
C)an electron
D)an alpha particle
A)a proton
B)a neutron
C)an electron
D)an alpha particle

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
19
The observation that 15.0 g of hydrogen reacts with 120.0 g of oxygen to form 135.0 g of water is evidence for the law of
A)definite proportions.
B)energy conservation.
C)mass conservation.
D)multiple proportions.
A)definite proportions.
B)energy conservation.
C)mass conservation.
D)multiple proportions.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
20
A sample of pure calcium fluoride with a mass of 15.0 g contains 7.70 g of calcium.How much calcium is contained in 45.0 g of calcium fluoride?
A)2)56 g
B)7)70 g
C)15.0 g
D)23.1 g
A)2)56 g
B)7)70 g
C)15.0 g
D)23.1 g

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
21
The element antimony has an atomic weight of 121.757 amu and only two naturally-occurring isotopes.One isotope has an abundance of 57.3% and an isotopic mass of 120.904 amu.Based on these data,what is the mass of the other isotope?
A)121.757 amu
B)122.393 amu
C)122.610 amu
D)122.902 amu
A)121.757 amu
B)122.393 amu
C)122.610 amu
D)122.902 amu

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
22
An element has two naturally occurring isotopes.One has an abundance of 37.4% and an isotopic mass of 184.953 amu,and the other has an abundance of 62.6% and a mass of 186.956 amu.What is the atomic weight of the element?
A)185.702 amu
B)185.954 amu
C)186.207 amu
D)186.956 amu
A)185.702 amu
B)185.954 amu
C)186.207 amu
D)186.956 amu

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
23
The number of atoms of carbon in 12 g of carbon is closest to .
A)12
B)1022
C)1023
D)1024
A)12
B)1022
C)1023
D)1024

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
24
The smallest sample of carbon atoms that can be observed with the naked eye has a mass of approximately 2 × 10-8 g.Given that 1 g = 6.02 × 1023 amu,and that carbon has an atomic weight of 12.01 amu,determine the number of carbon atoms present in the sample.
A)1 × 1015
B)1 × 1016
C)1 × 1017
D)6 × 1023
A)1 × 1015
B)1 × 1016
C)1 × 1017
D)6 × 1023

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
25
What is the chemical symbol for an atom that has 29 protons and 36 neutrons?
A)Cu
B)Kr
C)N
D)Tb
A)Cu
B)Kr
C)N
D)Tb

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
26
One mole of which element has the smallest mass?
A)Co
B)Cu
C)Ni
D)Zn
A)Co
B)Cu
C)Ni
D)Zn

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
27
How many protons (p)and neutrons (n)are in an atom of 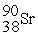
?
A)38 p,52 n
B)38 p,90 n
C)52 p,38 n
D)90 p,38 n
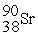
?
A)38 p,52 n
B)38 p,90 n
C)52 p,38 n
D)90 p,38 n

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
28
What is the mass of one atom of the element hydrogen?
A)2)0 g
B)1)0 g
C)3)4 × 10-24 g
D)1)7 × 10-24 g
A)2)0 g
B)1)0 g
C)3)4 × 10-24 g
D)1)7 × 10-24 g

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
29
How many protons (p)and neutrons (n)are in an atom of calcium-46?
A)20 p,26 n
B)20 p,46 n
C)26 p,20 n
D)46 p,60 n
A)20 p,26 n
B)20 p,46 n
C)26 p,20 n
D)46 p,60 n

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
30
How many protons (p),neutrons (n),and electrons (e)are in one atom of 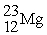
?
A)12 p,12 n,12 e
B)12 p,11 n,12 e
C)12 p,11 n,10 e
D)12 p,11 n,14 e
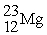
?
A)12 p,12 n,12 e
B)12 p,11 n,12 e
C)12 p,11 n,10 e
D)12 p,11 n,14 e

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
31
The atoms of a particular element all have the same number of protons as neutrons.Which of the following must be true?
A)The atomic weight must be a whole number.
B)The mass number for each atom must equal the atomic weight of the element.
C)The mass number must be exactly twice the atomic number for each atom.
D)All of these are true.
A)The atomic weight must be a whole number.
B)The mass number for each atom must equal the atomic weight of the element.
C)The mass number must be exactly twice the atomic number for each atom.
D)All of these are true.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following two atoms are isotopes?
A)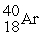 and
and 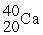
B)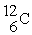 and
and 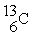
C)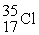 and
and 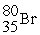
D)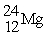 and
and 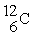
A)
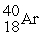 and
and 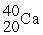
B)
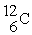 and
and 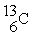
C)
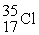 and
and 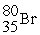
D)
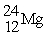 and
and 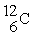

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
33
How many electrons are in a neutral atom of iodine-131?
A)1
B)53
C)54
D)131
A)1
B)53
C)54
D)131

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
34
24.0 g of which element contains the greatest number of atoms?
A)B
B)C
C)N
D)O
A)B
B)C
C)N
D)O

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
35
The isotope represented by 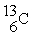
is named
A)carbon-6
B)carbon-7
C)carbon-13
D)carbon-19
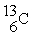
is named
A)carbon-6
B)carbon-7
C)carbon-13
D)carbon-19

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
36
What is the standard isotope that is used to define the number of atoms in a mole?
A)(1H)
B)(12C)
C)(16O)
D)(20Ne)
A)(1H)
B)(12C)
C)(16O)
D)(20Ne)

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
37
Boron-9 can be represented as
A)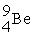 .
.
B)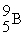 .
.
C)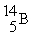 .
.
D)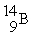 .
.
A)
 .
.B)
 .
.C)
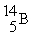 .
.D)
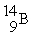 .
.
Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
38
Which are isotopes? An atom that has an atomic number of 34 and a mass number of 76 is an isotope of an atom that has
A)an atomic number of 32 and a mass number of 76.
B)an atomic number of 34 and a mass number of 80.
C)42 neutrons and 34 protons.
D)42 protons and 34 neutrons.
A)an atomic number of 32 and a mass number of 76.
B)an atomic number of 34 and a mass number of 80.
C)42 neutrons and 34 protons.
D)42 protons and 34 neutrons.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
39
Identify the chemical symbol of element Q in 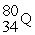
A)Br
B)Hg
C)Pd
D)Se
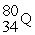
A)Br
B)Hg
C)Pd
D)Se

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following represent isotopes?
[A:![<strong>Which of the following represent isotopes? [A: ] [ B: ] [ C: ] [ D: ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4939/11ea7a38_c89f_9436_aa4c_0ba70ceda98c_TB4939_11.jpg) ]
]
[ B:![<strong>Which of the following represent isotopes? [A: ] [ B: ] [ C: ] [ D: ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4939/11ea7a38_c89f_bb47_aa4c_575c9617b6aa_TB4939_11.jpg) ]
]
[ C:![<strong>Which of the following represent isotopes? [A: ] [ B: ] [ C: ] [ D: ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4939/11ea7a38_c89f_bb48_aa4c_bdbe43af0171_TB4939_11.jpg) ]
]
[ D:![<strong>Which of the following represent isotopes? [A: ] [ B: ] [ C: ] [ D: ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4939/11ea7a38_c89f_bb49_aa4c_3385eb9c6fea_TB4939_11.jpg) ]
]
A)A and B
B)A and C
C)A and D
D)C and D
[A:
![<strong>Which of the following represent isotopes? [A: ] [ B: ] [ C: ] [ D: ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4939/11ea7a38_c89f_9436_aa4c_0ba70ceda98c_TB4939_11.jpg) ]
][ B:
![<strong>Which of the following represent isotopes? [A: ] [ B: ] [ C: ] [ D: ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4939/11ea7a38_c89f_bb47_aa4c_575c9617b6aa_TB4939_11.jpg) ]
][ C:
![<strong>Which of the following represent isotopes? [A: ] [ B: ] [ C: ] [ D: ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4939/11ea7a38_c89f_bb48_aa4c_bdbe43af0171_TB4939_11.jpg) ]
][ D:
![<strong>Which of the following represent isotopes? [A: ] [ B: ] [ C: ] [ D: ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4939/11ea7a38_c89f_bb49_aa4c_3385eb9c6fea_TB4939_11.jpg) ]
]A)A and B
B)A and C
C)A and D
D)C and D

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
41
The number of neutrons in 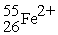
Is
A)26.
B)29.
C)53.
D)55.
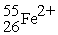
Is
A)26.
B)29.
C)53.
D)55.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
42
Positron emission changes the atomic number of an element by
A)-2.
B)-1.
C)+1.
D)+2.
A)-2.
B)-1.
C)+1.
D)+2.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following statements about gamma radiation is false?
A)It almost always accompanies alpha or beta emission.
B)It is a mechanism to release excess energy in the nucleus.
C)Gamma rays are high energy photons.
D)The mass number decreases by one with each gamma emitted.
A)It almost always accompanies alpha or beta emission.
B)It is a mechanism to release excess energy in the nucleus.
C)Gamma rays are high energy photons.
D)The mass number decreases by one with each gamma emitted.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
44
The number of nucleons in an atom or ion is the same as the
A)atomic number.
B)charge on the atom or ion.
C)mass number.
D)none of these
A)atomic number.
B)charge on the atom or ion.
C)mass number.
D)none of these

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following statements is not correct when balancing a nuclear equation?
I.The mass numbers must be conserved on both sides of the reaction arrow.
II.The ionic charges must be conserved on both sides of the reaction arrow.
III.The atomic numbers must be conserved on both sides of the reaction arrow.
IV.The elements must be the same on both sides of the reaction arrow.
A)II only
B)II and III
C)I and III
D)II and IV
I.The mass numbers must be conserved on both sides of the reaction arrow.
II.The ionic charges must be conserved on both sides of the reaction arrow.
III.The atomic numbers must be conserved on both sides of the reaction arrow.
IV.The elements must be the same on both sides of the reaction arrow.
A)II only
B)II and III
C)I and III
D)II and IV

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
46
The number of nucleons in a 
nucleus is
A)92.
B)144.
C)236.
D)328.

nucleus is
A)92.
B)144.
C)236.
D)328.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
47
A beta particle is
A)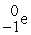 .
.
B)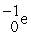 .
.
C)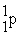 .
.
D)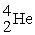 .
.
A)
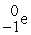 .
.B)
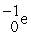 .
.C)
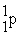 .
.D)
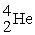 .
.
Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
48
A positron is
A)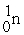 .
.
B)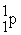 .
.
C)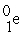 .
.
D)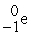 .
.
A)
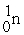 .
.B)
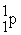 .
.C)
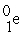 .
.D)
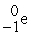 .
.
Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
49
Which statement about nuclear reactions is true?
A)New elements are never produced in a nuclear reaction.
B)Nuclear reactions involve valence electrons.
C)The rate of a nuclear reaction is affected by catalysts.
D)Tremendous amounts of energy are involved in nuclear reactions.
A)New elements are never produced in a nuclear reaction.
B)Nuclear reactions involve valence electrons.
C)The rate of a nuclear reaction is affected by catalysts.
D)Tremendous amounts of energy are involved in nuclear reactions.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
50
Gamma radiation can be described as
A)a helium nucleus.
B)a negatively charged free electron.
C)high energy electromagnetic radiation.
D)a positively charged free electron.
A)a helium nucleus.
B)a negatively charged free electron.
C)high energy electromagnetic radiation.
D)a positively charged free electron.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
51
The rate of a nuclear reaction can be changed by
A)adding a catalyst.
B)decreasing the pressure.
C)increasing the temperature.
D)None of these
A)adding a catalyst.
B)decreasing the pressure.
C)increasing the temperature.
D)None of these

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
52
The nuclear decay process that involves the particle having the greatest mass is ________ emission.
A)alpha
B)beta
C)gamma
D)positron
A)alpha
B)beta
C)gamma
D)positron

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
53
"Isotopes" are atoms with the same number of ________ but different number of ________.
A)electrons,protons
B)neutrons,protons
C)protons,electrons
D)protons,neutrons
A)electrons,protons
B)neutrons,protons
C)protons,electrons
D)protons,neutrons

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
54
When a substance decays by alpha radiation,the mass number of the nucleus ________ and the atomic number ________.
A)increases by 4,increases by 2
B)reduces by 4,reduces by 2
C)increases by 2,increases by 4
D)reduces by 2,reduces by 4
A)increases by 4,increases by 2
B)reduces by 4,reduces by 2
C)increases by 2,increases by 4
D)reduces by 2,reduces by 4

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
55
Beta decay of 24Na produces a beta particle and
A)(20F.)
B)(23Na.)
C)(24Ne.)
D)(24Mg.)
A)(20F.)
B)(23Na.)
C)(24Ne.)
D)(24Mg.)

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
56
When a substance decays by beta emission,the mass number of the nucleus ________ and the atomic number ________.
A)decreases by 1,remains the same
B)increases by 1,remains the same
C)remains the same,decreases by 1
D)remains the same,increases by 1
A)decreases by 1,remains the same
B)increases by 1,remains the same
C)remains the same,decreases by 1
D)remains the same,increases by 1

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
57
An alpha particle is
A)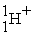 .
.
B)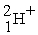 .
.
C)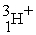 .
.
D)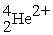 .
.
A)
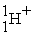 .
.B)
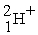 .
.C)
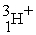 .
.D)
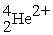 .
.
Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
58
How many moles and how many atoms of zinc are in a sample weighing 34.9 g?
A)0)533 mol,8.85 ×10-25 atoms
B)0)533 mol,3.21 ×1023 atoms
C)1)87 mol,3.10 × 10-24 atoms
D)1)87 mol,1.13 × 1024 atoms
A)0)533 mol,8.85 ×10-25 atoms
B)0)533 mol,3.21 ×1023 atoms
C)1)87 mol,3.10 × 10-24 atoms
D)1)87 mol,1.13 × 1024 atoms

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following statements about positrons is false?
A)The positron has same mass as an electron.
B)A positron is ejected from the nucleus during the conversion of a proton into a neutron.
C)A positron is a positive electron.
D)When positron emission occurs,the atomic number of the nucleus increases.
A)The positron has same mass as an electron.
B)A positron is ejected from the nucleus during the conversion of a proton into a neutron.
C)A positron is a positive electron.
D)When positron emission occurs,the atomic number of the nucleus increases.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
60
The term "nucleons" refers to the number of ________ in the atom.
A)neutrons
B)protons
C)protons and neutrons
D)protons,neutrons,and electrons
A)neutrons
B)protons
C)protons and neutrons
D)protons,neutrons,and electrons

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
61
Which is the only element that contains more protons than neutrons in its most abundant stable isotope?
A)boron
B)carbon
C)hydrogen
D)mercury
A)boron
B)carbon
C)hydrogen
D)mercury

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
62
Which one of the following combinations of neutrons/protons results in the lowest number of nonradioactive (stable)isotopes?
A)even number protons/even number neutrons
B)even number protons/odd number neutrons
C)odd number protons/even number neutrons
D)odd number protons/odd number neutrons
A)even number protons/even number neutrons
B)even number protons/odd number neutrons
C)odd number protons/even number neutrons
D)odd number protons/odd number neutrons

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
63
Which one of the following processes does not result in transmutation to another element?
A)alpha emission
B)beta emission
C)electron capture
D)gamma emission
A)alpha emission
B)beta emission
C)electron capture
D)gamma emission

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following elements would you expect to have the largest number of stable isotopes? Element number:
A)48
B)49
C)50
D)51
A)48
B)49
C)50
D)51

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
65
In addition to a beta particle,what is the other product of beta decay of 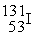
?
A)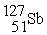
B)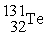
C)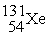
D)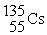
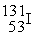
?
A)
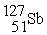
B)
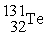
C)
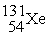
D)
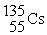

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following statements about electron capture is false?
A)The electron is used to convert a proton to a neutron.
B)The electron involved is most likely an outer shell valence electron.
C)In electron capture decay,the atomic number decreases by one.
D)In electron capture decay,the mass number remains unchanged.
A)The electron is used to convert a proton to a neutron.
B)The electron involved is most likely an outer shell valence electron.
C)In electron capture decay,the atomic number decreases by one.
D)In electron capture decay,the mass number remains unchanged.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
67
Tritium,  ,is formed in the upper atmosphere when
,is formed in the upper atmosphere when  Captures a neutron and then decays.What is the other product of this reaction?
Captures a neutron and then decays.What is the other product of this reaction?
A)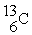
B)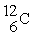
C)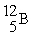
D)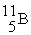
 ,is formed in the upper atmosphere when
,is formed in the upper atmosphere when  Captures a neutron and then decays.What is the other product of this reaction?
Captures a neutron and then decays.What is the other product of this reaction?A)
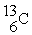
B)
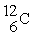
C)
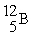
D)
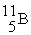

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
68
When more than 3000 known nuclides are plotted on a neutron/proton grid they make up a group called
A)the "island of stability."
B)the "peninsula of nuclear stability."
C)the "sea of instability."
D)none of these
A)the "island of stability."
B)the "peninsula of nuclear stability."
C)the "sea of instability."
D)none of these

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
69
A radioisotope which is neutron poor and very heavy is most likely to decay by
A)alpha emission,electron capture,or positron emission.
B)only alpha emission.
C)only electron capture.
D)only positron emission.
A)alpha emission,electron capture,or positron emission.
B)only alpha emission.
C)only electron capture.
D)only positron emission.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
70
Which reaction below represents 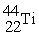 Decay by electron capture?
Decay by electron capture?
A)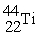 +
+ 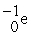 →
→ 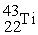
B)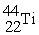 +
+ 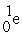 →
→ 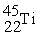
C)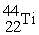 +
+ 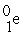 →
→ 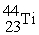
D)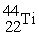 +
+ 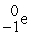 →
→ 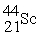
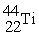 Decay by electron capture?
Decay by electron capture?A)
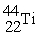 +
+ 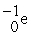 →
→ 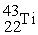
B)
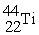 +
+ 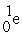 →
→ 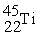
C)
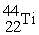 +
+ 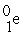 →
→ 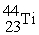
D)
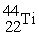 +
+ 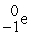 →
→ 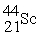

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
71
Which one of the following statements about isotopes is false?
A)The ratio of neutrons to protons is about 1:1 for elements lighter than Ca.
B)The ratio of neutrons to protons is > 1:1 for elements heavier than Ca.
C)Nonradioactive isotopes generally have an odd number of neutrons.
D)All isotopes beyond 209Bi are radioactive.
A)The ratio of neutrons to protons is about 1:1 for elements lighter than Ca.
B)The ratio of neutrons to protons is > 1:1 for elements heavier than Ca.
C)Nonradioactive isotopes generally have an odd number of neutrons.
D)All isotopes beyond 209Bi are radioactive.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
72
Which reaction below represents 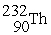 Decay by alpha emission?
Decay by alpha emission?
A)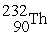 →
→ 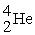 +
+ 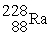
B)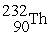 →
→ 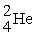 +
+ 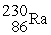
C)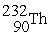 →
→ 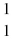 p +
p + 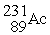
D)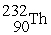 →
→ 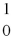 n +
n + 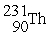
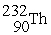 Decay by alpha emission?
Decay by alpha emission?A)
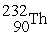 →
→ 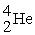 +
+ 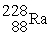
B)
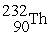 →
→ 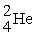 +
+ 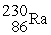
C)
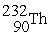 →
→ 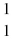 p +
p + 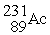
D)
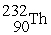 →
→ 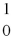 n +
n + 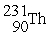

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
73
Which process decreases the neutron/proton ratio?
A)alpha emission
B)beta emission
C)electron capture
D)positron emission
A)alpha emission
B)beta emission
C)electron capture
D)positron emission

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
74
Which reaction below represents 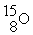 Decay by positron emission?
Decay by positron emission?
A)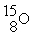 →
→ 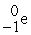 +
+ 
B)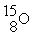 →
→ 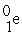 +
+ 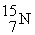
C)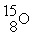 →
→ 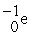 +
+ 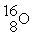
D)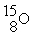 →
→ 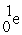 +
+ 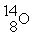
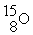 Decay by positron emission?
Decay by positron emission?A)
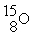 →
→ 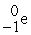 +
+ 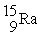
B)
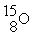 →
→ 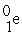 +
+ 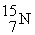
C)
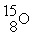 →
→ 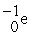 +
+ 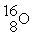
D)
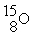 →
→ 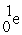 +
+ 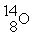

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
75
The nuclear transformation potassium-40 argon-40 + ? is classified as
A)alpha emission.
B)beta emission.
C)electron capture.
D)positron emission.
A)alpha emission.
B)beta emission.
C)electron capture.
D)positron emission.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following nuclides is most likely to undergo beta decay?
A)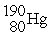
B)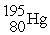
C)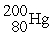
D)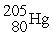
A)
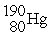
B)
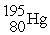
C)
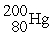
D)
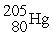

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
77
As the atomic number of the elements increases,the ratio of neutrons to protons in stable nuclei
A)decreases.
B)stays the same.
C)increases.
D)is unrelated to stability.
A)decreases.
B)stays the same.
C)increases.
D)is unrelated to stability.

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following decay processes give a product nuclide whose atomic number is one less than the starting nuclide?
A)alpha decay
B)beta decay and positron decay
C)gamma decay and beta decay
D)positron decay and electron capture
A)alpha decay
B)beta decay and positron decay
C)gamma decay and beta decay
D)positron decay and electron capture

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following elements would be expected to be particularly stable?
A)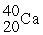
B)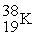
C)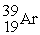
D)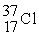
A)
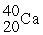
B)
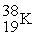
C)
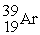
D)
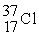

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck
80
A radioisotope has a neutron/proton ratio which is too low.Which of the following processes will not occur for such a nucleus?
A)alpha emission
B)beta emission
C)electron capture
D)positron emission
A)alpha emission
B)beta emission
C)electron capture
D)positron emission

Unlock Deck
Unlock for access to all 257 flashcards in this deck.
Unlock Deck
k this deck


