Exam 2: Atoms, molecules, and Ions
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
A sample of pure lithium carbonate contains 18.8% lithium by mass.What is the % lithium by mass in a sample of pure lithium carbonate that has twice the mass of the first sample?
Free
(Multiple Choice)
4.8/5  (50)
(50)
Correct Answer:
B
Which element can form more than one kind of monatomic ion?
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
C
Use the periodic table below to answer the following questions. 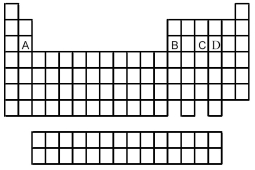 -Which is the correct formula of the binary fluoride of element A?
-Which is the correct formula of the binary fluoride of element A?
(Multiple Choice)
4.8/5  (38)
(38)
Methane and oxygen react to form carbon dioxide and water.What mass of water is formed if 3.2 g of methane reacts with 12.8 g of oxygen to produce 8.8 g of carbon dioxide?
(Multiple Choice)
4.9/5  (35)
(35)
Use the periodic table below to answer the following questions. 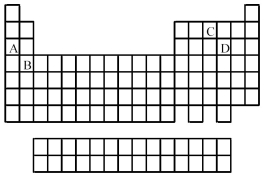 -Which elements commonly form anions?
-Which elements commonly form anions?
(Multiple Choice)
4.7/5  (37)
(37)
In addition to a beta particle,what is the other product of beta decay of 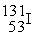 ?
?
(Multiple Choice)
4.8/5  (33)
(33)
A sample of pure calcium fluoride with a mass of 15.0 g contains 7.70 g of calcium.How much calcium is contained in 40.0 g of calcium fluoride?
(Multiple Choice)
4.7/5  (35)
(35)
The observation that hydrogen and oxygen can react to form two compounds with different chemical and physical properties,one having an O:H mass ratio = 8:1 and the other having an O:H mass ratio = 16:1 is consistent with the law of
(Multiple Choice)
4.8/5  (34)
(34)
In the following drawings,shaded spheres represent cations and unshaded spheres represent anions. 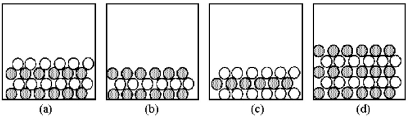 -Which drawing represents the ionic compound CaCl2?
-Which drawing represents the ionic compound CaCl2?
(Multiple Choice)
4.9/5  (36)
(36)
If shaded and unshaded spheres represent atoms of different elements,as shown in drawing (1),which drawings (2)-(4)represent the law of multiple proportions? 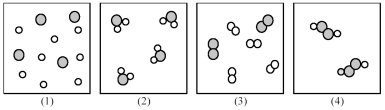
(Multiple Choice)
4.9/5  (34)
(34)
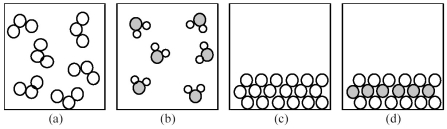 -If shaded and unshaded spheres represent atoms of different elements,which of the above drawings most likely represents an ionic compound at room temperature and a pressure of 1 atm?
-If shaded and unshaded spheres represent atoms of different elements,which of the above drawings most likely represents an ionic compound at room temperature and a pressure of 1 atm?
(Multiple Choice)
4.7/5  (37)
(37)
A sample of pure calcium fluoride with a mass of 15.0 g contains 7.70 g of calcium.How much calcium is contained in 45.0 g of calcium fluoride?
(Multiple Choice)
5.0/5  (38)
(38)
What is the identity of element Q if the ion Q2+ contains 10 electrons?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following statements about gamma radiation is false?
(Multiple Choice)
4.7/5  (31)
(31)
Which of the following is a part of Dalton's atomic theory?
(Multiple Choice)
4.9/5  (42)
(42)
Showing 1 - 20 of 257
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)