Deck 9: Gases: Their Properties and Behavior
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/182
Play
Full screen (f)
Deck 9: Gases: Their Properties and Behavior
1
An approximation of absolute zero was made from an extrapolation of
A)P vs.1/V
B)V vs.T
C)n vs.V
D)V vs.1/T
A)P vs.1/V
B)V vs.T
C)n vs.V
D)V vs.1/T
V vs.T
2
The pressure in the eye of a hurricane is less than atmospheric pressure.Which one of the following pressure readings could not have been taken in the eye of a hurricane?
A)15 lbs/in2
B)69 cm Hg
C)690 mm Hg
D)9)22 × 104 Pa
A)15 lbs/in2
B)69 cm Hg
C)690 mm Hg
D)9)22 × 104 Pa
15 lbs/in2
3
What is the pressure in a gas container that is connected to an open-end U-tube manometer if the pressure of the atmosphere is 752 torr and the level of mercury in the arm connected to the container is 8.60 cm higher than the level of mercury open to the atmosphere?
A)666 mm Hg
B)743 mm Hg
C)761 mm Hg
D)838 mm Hg
A)666 mm Hg
B)743 mm Hg
C)761 mm Hg
D)838 mm Hg
666 mm Hg
4
Which of the following instruments directly measures the pressure of a gas?
A)spectrometer
B)manometer
C)polarimeter
D)gas chromatograph
A)spectrometer
B)manometer
C)polarimeter
D)gas chromatograph

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following equations represents "Boyle's law"?
A)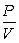 = k
= k
B)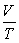 = k
= k
C)PV = k
D)V = nk
A)
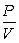 = k
= kB)
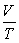 = k
= kC)PV = k
D)V = nk

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
6
If mercury (density = 13.6 g/cm3)at a height of 745 mm Hg in a mercury barometer is replaced with water (density = 1.00 g/cm3),under the same conditions the height of water will be
A)0)180 ft
B)2)44 ft
C)33.2 ft
D)399 ft
A)0)180 ft
B)2)44 ft
C)33.2 ft
D)399 ft

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
7
If the pressure in a gas container that is connected to an open-end U-tube manometer is 106 kPa and the pressure of the atmosphere at the open end of the tube is 742 mm Hg,the level of mercury in the tube will be
A)53 mm higher in the arm open to the atmosphere.
B)53 mm higher in the arm connected to the gas cylinder.
C)636 mm higher in the arm open to the atmosphere.
D)636 mm higher in the arm connected to the gas cylinder.
A)53 mm higher in the arm open to the atmosphere.
B)53 mm higher in the arm connected to the gas cylinder.
C)636 mm higher in the arm open to the atmosphere.
D)636 mm higher in the arm connected to the gas cylinder.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
8
Automobile tires are typically inflated to about 30 pounds of pressure per square inch.What is the typical air pressure of a tire in kPa?
A)2)0 × 10-3 kPa
B)2)0 kPa
C)2)1 × 102 kPa
D)2)1 × 105 kPa
A)2)0 × 10-3 kPa
B)2)0 kPa
C)2)1 × 102 kPa
D)2)1 × 105 kPa

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
9
Which is the smallest quantity of pressure?
A)1 atm
B)1 centimeter of Hg
C)1 mm Hg
D)1 pascal
A)1 atm
B)1 centimeter of Hg
C)1 mm Hg
D)1 pascal

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
10
When temperature-volume measurements are made on 1.0 mol of gas at 1.0 atm,a plot V versus T results in a
A)hyperbola.
B)parabola.
C)sine curve.
D)straight line.
A)hyperbola.
B)parabola.
C)sine curve.
D)straight line.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
11
In an open end manometer,one end of a U-tube filled with mercury is attached to a gas-filled container and the other end is open to the atmosphere.If the gas pressure in the container is less than atmospheric pressure
A)Hg will be forced out of the open end of the U-tube.
B)the difference between the Hg levels in the two arms will be greater than 76 cm.
C)the Hg level will be higher in the arm connected to the container.
D)the Hg level will be higher in the arm open to the atmosphere.
A)Hg will be forced out of the open end of the U-tube.
B)the difference between the Hg levels in the two arms will be greater than 76 cm.
C)the Hg level will be higher in the arm connected to the container.
D)the Hg level will be higher in the arm open to the atmosphere.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
12
The SI unit for pressure is the
A)atmosphere.
B)MM Hg.
C)newton.
D)pascal.
A)atmosphere.
B)MM Hg.
C)newton.
D)pascal.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
13
When pressure-volume measurements are made on 1.0 mol of gas at constant temperature,a plot V versus P results in a
A)hyperbola.
B)parabola.
C)sine curve.
D)straight line.
A)hyperbola.
B)parabola.
C)sine curve.
D)straight line.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
14
Suppose you needed to closely monitor small changes in pressure inside a container using an open end manometer.For the best accuracy,the substance in the manometer should
A)be a solid.
B)be mercury.
C)have a high density.
D)have a low density.
A)be a solid.
B)be mercury.
C)have a high density.
D)have a low density.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
15
Carbon dioxide is a gas which causes environmental concern because of the greenhouse effect.What is the approximate percentage (by volume)of CO2 in the atmosphere?
A)less than 0.1%
B)about 1%
C)about 10%
D)more than 20%
A)less than 0.1%
B)about 1%
C)about 10%
D)more than 20%

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
16
A container filled with gas is connected to an open-end U-tube manometer that is filled with mineral oil.The pressure in the gas container is 773 mm Hg and atmospheric pressure is 754 mm Hg.What will be the difference in the levels of mineral oil in the two arms of the manometer if the densities of Hg and mineral oil are 13.6 g/mL and 0.822 g/mL respectively?
A)1)15 mm
B)15.6 mm
C)19.0 mm
D)314 mm
A)1)15 mm
B)15.6 mm
C)19.0 mm
D)314 mm

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
17
Which law does the equation, 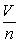
= k represent?
A)Avogadro's law
B)Boyle's law
C)Charles' law
D)Graham's law
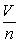
= k represent?
A)Avogadro's law
B)Boyle's law
C)Charles' law
D)Graham's law

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
18
Pressure is defined as
A)force divided by unit area.
B)force times unit area.
C)mass divided by acceleration.
D)mass times acceleration.
A)force divided by unit area.
B)force times unit area.
C)mass divided by acceleration.
D)mass times acceleration.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is not equivalent to 1 atm pressure?
A)10 cm Hg
B)14.7 lb/in2
C)101 kPa
D)760 mm Hg
A)10 cm Hg
B)14.7 lb/in2
C)101 kPa
D)760 mm Hg

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
20
Which one of the following is not used to describe the condition of a gas?
A)number of moles
B)polarity
C)temperature
D)volume
A)number of moles
B)polarity
C)temperature
D)volume

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
21
What is the Celsius temperature of 100.0 g of chlorine gas in a 40.0-L container at 800 mm Hg?
A)-91°C
B)91°C
C)182°C
D)364°C
A)-91°C
B)91°C
C)182°C
D)364°C

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
22
A basketball is inflated to a pressure of 1.50 atm in a 20.0°C garage.What is the pressure of the basketball outside where the temperature is -5.00°C?
A)1)37 atm
B)1)42 atm
C)1)58 atm
D)1)64 atm
A)1)37 atm
B)1)42 atm
C)1)58 atm
D)1)64 atm

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
23
Cyanogen is a gas which contains 46.2% C and 53.8% N by mass.At a temperature of 25°C and a pressure of 750 mm Hg,1.50 g of cyanogen occupies 0.714 L.What is the molecular formula of cyanogen?
A)CN
B)C2N2
C)C3N4
D)C4N5
A)CN
B)C2N2
C)C3N4
D)C4N5

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
24
The volume of 350.mL of gas at 25°C is decreased to 125 mL at constant pressure.What is the final temperature of the gas?
A)-167°C
B)8)9°C
C)70°C
D)561°C
A)-167°C
B)8)9°C
C)70°C
D)561°C

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
25
A gas occupies 22.4 L at STP and 14.5 L at 100°C and 2.00 atm pressure.How many moles of gas did the system gain or lose?
A)0)06 moles gained
B)0)03 moles gained
C)0)03 moles lost
D)0)05 moles lost
A)0)06 moles gained
B)0)03 moles gained
C)0)03 moles lost
D)0)05 moles lost

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
26
A 1.75 L container filled with CO2 gas at 25°C and 225 kPa pressure springs a leak.When the container is re-sealed,the pressure is 185 kPA and the temperature is 10°C.How many moles of gas were lost?
A)0)0213 mol
B)0)463 mol
C)0)561 mol
D)2)16 mol
A)0)0213 mol
B)0)463 mol
C)0)561 mol
D)2)16 mol

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
27
A balloon filled with helium gas at 20°C occupies 2.91 L at 1.00 atm.The balloon is immersed in liquid nitrogen at -196°C,raising the pressure to 5.20 atm.What is the volume of the balloon in the liquid nitrogen?
A)0)15 L
B)2)1 L
C)4)0 L
D)58 L
A)0)15 L
B)2)1 L
C)4)0 L
D)58 L

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
28
If the number of moles of gas is doubled at constant temperature and volume,the pressure of the gas
A)is halved.
B)is doubled.
C)is quadrupled.
D)remains the same.
A)is halved.
B)is doubled.
C)is quadrupled.
D)remains the same.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
29
A 1.00 L flask contains nitrogen gas at 25°C and 1.00 atm pressure.What is the final pressure in the flask if an additional 2.00 g of N2 gas is added to the flask and the flask cooled to -55°C?
A)1)28 atm
B)2)01 atm
C)2)56 atm
D)3)29 atm
A)1)28 atm
B)2)01 atm
C)2)56 atm
D)3)29 atm

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
30
A steel bottle contains argon gas at STP.What is the final pressure if the temperature is changed to 115°C?
A)0)704 atm
B)0)768 atm
C)1)30 atm
D)1)42 atm
A)0)704 atm
B)0)768 atm
C)1)30 atm
D)1)42 atm

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
31
How many grams of O2 gas are there in a 5.00-L cylinder at 4.00 × 103 mm Hg and 23°C?
A)17.3 g
B)34.7 g
C)446 g
D)2)63 × 104 g
A)17.3 g
B)34.7 g
C)446 g
D)2)63 × 104 g

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
32
How many molecules of N2 are in a 500.0 mL container at 780 mm Hg and 135°C?
A)8)76 × 1021
B)9)23 × 1021
C)2)65 × 1022
D)2)79 × 1022
A)8)76 × 1021
B)9)23 × 1021
C)2)65 × 1022
D)2)79 × 1022

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
33
A 75.0 L steel tank at 20.0°C contains acetylene gas,C2H2,at a pressure of 1.39 atm.Assuming ideal behavior,how many grams of acetylene are in the tank?
A)4)33 g
B)6)01 g
C)113 g
D)1650 g
A)4)33 g
B)6)01 g
C)113 g
D)1650 g

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
34
Three identical flasks contain three different gases at standard temperature and pressure.Flask A contains CH4,flask B contains CO2,flask C contains N2.Which flask contains the largest number of molecules?
A)flask A
B)flask B
C)flask C
D)All flasks contain the same number of molecules.
A)flask A
B)flask B
C)flask C
D)All flasks contain the same number of molecules.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
35
"Equal volumes of different gases at the same temperature and pressure contain the same molar amounts" is another way of stating
A)Avogadro's law.
B)Boyle's law.
C)Charles' law.
D)Graham's law.
A)Avogadro's law.
B)Boyle's law.
C)Charles' law.
D)Graham's law.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
36
What is the value of the gas constant,R,in units of 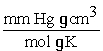
?
A)1)080 × 10-4
B)0)1080
C)62.36
D)6)236 × 104
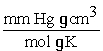
?
A)1)080 × 10-4
B)0)1080
C)62.36
D)6)236 × 104

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
37
An "empty" aerosol can at 25°C still contains gas at 1.00 atmosphere pressure.If an "empty" can is thrown into a 475°C fire,what is the final pressure in the heated can?
A)5)26 × 10-2 atm
B)0)398 atm
C)2)51 atm
D)19.0 atm
A)5)26 × 10-2 atm
B)0)398 atm
C)2)51 atm
D)19.0 atm

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
38
A 0.286-g sample of gas occupies 125 mL at 60.cm of Hg and 25°C.What is the molar mass of the gas?
A)5)9 g/mol
B)44 g/mol
C)59 g/mol
D)71 g/mol
A)5)9 g/mol
B)44 g/mol
C)59 g/mol
D)71 g/mol

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
39
A gas bottle contains 0.650 mol of gas at 730 mm Hg pressure.If the final pressure is 1.15 atm,how many moles of gas were added to the bottle?
A)0)0680 mol
B)0)128 mol
C)0)717 mol
D)0)778 mol
A)0)0680 mol
B)0)128 mol
C)0)717 mol
D)0)778 mol

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
40
What is the volume of 10.0 g of argon gas at 157°C and 2.50 kPa pressure?
A)1)29 L
B)3)53 L
C)131 L
D)358 L
A)1)29 L
B)3)53 L
C)131 L
D)358 L

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
41
How many liters of SO3(g)are produced at 25°C and 1.00 atm from the combustion of 1.00 kg of coal which is 1.00% S by weight? Assume all the sulfur in the coal ends up as SO3.
A)0)640 L
B)5)08 L
C)7)63 L
D)11.4 L
A)0)640 L
B)5)08 L
C)7)63 L
D)11.4 L

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
42
The action of some commercial drain cleaners is based on the following reaction:
2 NaOH(s)+ 2 Al(s)+ 6 H2O(l)→ 2 NaAl(OH)4(s)+ 3 H2(g)
What is the volume of H2 gas formed at STP when 4.32 g of Al reacts with excess NaOH?
A)2)39 L
B)3)59 L
C)5)38 L
D)5)87 L
2 NaOH(s)+ 2 Al(s)+ 6 H2O(l)→ 2 NaAl(OH)4(s)+ 3 H2(g)
What is the volume of H2 gas formed at STP when 4.32 g of Al reacts with excess NaOH?
A)2)39 L
B)3)59 L
C)5)38 L
D)5)87 L

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
43
Chloroform is a volatile liquid once commonly used in the laboratory but now being phased out due to its ozone depletion potential.If the pressure of gaseous chloroform in a flask is 195 mm Hg at 25°C and its density is 1.25 g/L,what is the molar mass of chloroform?
A)10.0 g/mol
B)76.3 g/mol
C)119 g/mol
D)None of these
A)10.0 g/mol
B)76.3 g/mol
C)119 g/mol
D)None of these

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
44
Hydrogen gas is collected over water in an inverted buret.If the atmospheric pressure is 745 mm Hg,the vapor pressure of water is 18 mm Hg,and a 15.0 cm-high column of water remains in the buret,the pressure of the hydrogen gas is
A)763 mm.
B)745 mm Hg.
C)727 mm Hg.
D)less than 727 mm Hg.
A)763 mm.
B)745 mm Hg.
C)727 mm Hg.
D)less than 727 mm Hg.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
45
What is the density of fluorine gas at STP?
A)0)590 g/L
B)0)848 g/L
C)1)55 g/L
D)1)70 g/L
A)0)590 g/L
B)0)848 g/L
C)1)55 g/L
D)1)70 g/L

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
46
How many liters of oxygen are needed to exactly react with 27.8 g of methane at STP?
CH4(g)+ 2 O2(g)→ CO2(g)+ 2 H2O(l)
A)19.5 L
B)39.0 L
C)77.6 L
D)85.0 L
CH4(g)+ 2 O2(g)→ CO2(g)+ 2 H2O(l)
A)19.5 L
B)39.0 L
C)77.6 L
D)85.0 L

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
47
Given three cylinders containing O2 gas at the same volume and pressure.Cylinder A is at -20°C,cylinder B is at -15°F,cylinder C is at 260 K.Which cylinder contains the largest mass of oxygen?
A)cylinder A
B)cylinder B
C)cylinder C
D)All cylinders contain the same mass of O2.
A)cylinder A
B)cylinder B
C)cylinder C
D)All cylinders contain the same mass of O2.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
48
At STP how many liters of NH3 can be produced from the reaction of 6.00 mol of N2 with 6.00 mol of H2?
N2(g)+ 3 H2(g)→ 2 NH3(g)
A)44.8 L
B)89.6 L
C)134 L
D)269 L
N2(g)+ 3 H2(g)→ 2 NH3(g)
A)44.8 L
B)89.6 L
C)134 L
D)269 L

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
49
In the laboratory,hydrogen gas is usually made by the following reaction:
Zn(s)+ 2 HCl(aq)→ H2(g)+ ZnCl2(aq)
How many liters of H2 gas,collected over water at an atmospheric pressure of 752 mm Hg and a temperature of 21.0°C,can be made from 1.566 g of Zn and excess HCl? The partial pressure of water vapor is 18.65 mm Hg at 21.0°C.
A)0)0428 L
B)0)573 L
C)0)585 L
D)0)599 L
Zn(s)+ 2 HCl(aq)→ H2(g)+ ZnCl2(aq)
How many liters of H2 gas,collected over water at an atmospheric pressure of 752 mm Hg and a temperature of 21.0°C,can be made from 1.566 g of Zn and excess HCl? The partial pressure of water vapor is 18.65 mm Hg at 21.0°C.
A)0)0428 L
B)0)573 L
C)0)585 L
D)0)599 L

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following would have a density of 1.21 g/L at 7.0°C and 0.987 atm?
A)Ar
B)N2
C)Ne
D)O2
A)Ar
B)N2
C)Ne
D)O2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
51
A lungful of air (500 mL)contains 4.1% CO2 by volume.How many grams of KO2(s)is needed to remove the CO2 from a lungful of air at STP according to the following reaction?
4 KO2(s)+ 2 CO2(g)→ 2 K2CO3(s)+ 3 O2(g)
A)0)065 g
B)0)13 g
C)0)26 g
D)1)2 g
4 KO2(s)+ 2 CO2(g)→ 2 K2CO3(s)+ 3 O2(g)
A)0)065 g
B)0)13 g
C)0)26 g
D)1)2 g

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
52
A balloon contains 0.76 mol N2,0.18 mol O2,0.031 mol He,and 0.026 mol H2 at 739 mm Hg.What is the partial pressure of O2?
A)19 mm Hg
B)23 mm Hg
C)130 mm Hg
D)560 mm Hg
A)19 mm Hg
B)23 mm Hg
C)130 mm Hg
D)560 mm Hg

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
53
A 10.0-L flask containing He,2.00 mole of Ar,and 3.00 mole of Ne has a total pressure of 24.5 atm at 25°C.How many moles of He are in the flask?
A)5)00 mol
B)10.0 mol
C)114 mol
D)119 mol
A)5)00 mol
B)10.0 mol
C)114 mol
D)119 mol

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
54
One mole of which gas has the greatest density at STP?
A)Ar
B)N2
C)CO
D)All three gases have the same density.
A)Ar
B)N2
C)CO
D)All three gases have the same density.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
55
When 15.0 g of zinc metal reacts with excess HCl,how many liters of H2 gas are produced at STP?
A)0)229 L
B)0)458 L
C)5)14 L
D)10.3 L
A)0)229 L
B)0)458 L
C)5)14 L
D)10.3 L

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
56
If the Earth's ozone (O3)layer has a total volume of 1.00 × 1020 km3,a partial pressure of 1.6 × 10-9 atm,and an average temperature of 230 K,how many ozone molecules are in the Earth's ozone layer?
A)2)3 × 1035 molecules
B)5)1 × 1035 molecules
C)2)3 × 1045 molecules
D)5)1 × 1045 molecules
A)2)3 × 1035 molecules
B)5)1 × 1035 molecules
C)2)3 × 1045 molecules
D)5)1 × 1045 molecules

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
57
A 1.000 kg sample of nitroglycerine,C3H5N3O9,explodes and releases gases with a temperature of 1985°C at 1.000 atm.What is the volume of gas produced?
4 C3H5N3O9(l)→ 12 CO2(g)+ 10 H2O(g)+ 6 N2(g)+ O2(g)
A)816.4 L
B)3878 L
C)5203 L
D)5919 L
4 C3H5N3O9(l)→ 12 CO2(g)+ 10 H2O(g)+ 6 N2(g)+ O2(g)
A)816.4 L
B)3878 L
C)5203 L
D)5919 L

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
58
At STP how many grams of Mg are required to produce 35 mL of H2 in the reaction shown below?
Mg(s)+ 2 HCl(aq)→ H2(g)+ MgCl2(aq)
A)0)035 g
B)0)038 g
C)26 g
D)29 g
Mg(s)+ 2 HCl(aq)→ H2(g)+ MgCl2(aq)
A)0)035 g
B)0)038 g
C)26 g
D)29 g

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
59
What is the total pressure in a 10.0 L flask which contains 0.127 mol of H2(g)and 0.288 mol of N2(g)at 20.0°C?
A)0)306 atm
B)0)681 atm
C)0)693 atm
D)0)998 atm
A)0)306 atm
B)0)681 atm
C)0)693 atm
D)0)998 atm

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
60
How many grams of XeF6 are required to react with 0.579 L of hydrogen gas at 2.46 atm and 45°C in the reaction shown below?
XeF6(s)+ 3 H2(g)→ Xe(g)+ 6 HF(g)
A)3)65 g
B)4)46 g
C)13.4 g
D)40.2 g
XeF6(s)+ 3 H2(g)→ Xe(g)+ 6 HF(g)
A)3)65 g
B)4)46 g
C)13.4 g
D)40.2 g

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
61
Each of three identical 15.0-L gas cylinders contains 7.50 mol of gas at 295 K.Cylinder A contains Ar,cylinder B contains Cl2,and cylinder C contains N2.According to the kinetic molecular theory,which gas has the highest density?
A)Ar
B)Cl2
C)N2
D)All have identical densities
A)Ar
B)Cl2
C)N2
D)All have identical densities

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
62
The ozone molecules in the stratosphere absorb much of the ultraviolet radiation from the sun,protecting life on Earth.At a certain altitude,the temperature of the stratosphere is 240 K and the partial pressure of ozone is 1.4 × 10-7 atm.Calculate the number of ozone molecules present in 1.00 L of atmosphere at that altitude.
A)2)1 × 1015 molecules of O3
B)4)3 × 1015 molecules of O3
C)8)0 × 1031 molecules of O3
D)1)8 × 1032 molecules of O3
A)2)1 × 1015 molecules of O3
B)4)3 × 1015 molecules of O3
C)8)0 × 1031 molecules of O3
D)1)8 × 1032 molecules of O3

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
63
According to the kinetic molecular theory,the pressure of a gas in a container will decrease if the
A)number of collisions with the container wall increases.
B)number of moles of the gas increases.
C)temperature of the gas decreases.
D)volume of the container decreases.
A)number of collisions with the container wall increases.
B)number of moles of the gas increases.
C)temperature of the gas decreases.
D)volume of the container decreases.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
64
The mixing of different gases by random molecular motion with frequent collisions is called
A)Avogadro's law.
B)compressibility.
C)diffusion.
D)effusion.
A)Avogadro's law.
B)compressibility.
C)diffusion.
D)effusion.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
65
A 0.500 g sample containing Ag2O and inert material is heated,causing the silver oxide to decompose according to the following equation:
2 Ag2O(s)→ 4 Ag(s)+ O2(g)
If 13.8 mL of gas are collected over water at 27°C and 1.00 atm external pressure,what is the percentage of silver oxide in the sample? The partial pressure of water is 26.7 mm Hg at 27°C.
A)12.5%
B)25.1%
C)50.1%
D)51.9%
2 Ag2O(s)→ 4 Ag(s)+ O2(g)
If 13.8 mL of gas are collected over water at 27°C and 1.00 atm external pressure,what is the percentage of silver oxide in the sample? The partial pressure of water is 26.7 mm Hg at 27°C.
A)12.5%
B)25.1%
C)50.1%
D)51.9%

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
66
What is the average speed (actually the root-mean-square speed)of a neon atom at 27°C?
A)5)78 m/s
B)19.3 m/s
C)183 m/s
D)609 m/s
A)5)78 m/s
B)19.3 m/s
C)183 m/s
D)609 m/s

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
67
Each of three identical 15.0-L gas cylinders contains 7.50 mol of gas at 295 K.Cylinder A contains Ar,cylinder B contains Cl2,and cylinder C contains N2.According to the kinetic molecular theory,which gas has the highest average speed?
A)Ar
B)Cl2
C)N2
D)All have identical average speeds
A)Ar
B)Cl2
C)N2
D)All have identical average speeds

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
68
What is the temperature of CO2 gas if the average speed (actually the root-mean-square speed)of the molecules is 750 m/s?
A)1)32 K
B)9)92 × 102 K
C)1)31 × 103 K
D)9)92 × 105 K
A)1)32 K
B)9)92 × 102 K
C)1)31 × 103 K
D)9)92 × 105 K

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
69
Each of three identical 15.0-L gas cylinders contains 7.50 mol of gas at 295 K.Cylinder A contains Ar,cylinder B contains Cl2,and cylinder C contains N2.According to the kinetic molecular theory,which gas has the highest pressure?
A)Ar
B)Cl2
C)N2
D)All have identical pressures
A)Ar
B)Cl2
C)N2
D)All have identical pressures

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
70
Some assumptions from the kinetic molecular theory are listed below.Which one is most frequently cited to explain diffusion of a gas?
A)The average kinetic energy of gas particles is proportional to the Kelvin temperature.
B)Collisions of gas particles are elastic and total kinetic energy of the gas is constant.
C)A gas consist of tiny particles moving in random straight line motion.
D)The volume of the particles is negligible compared to the volume of the gas.
A)The average kinetic energy of gas particles is proportional to the Kelvin temperature.
B)Collisions of gas particles are elastic and total kinetic energy of the gas is constant.
C)A gas consist of tiny particles moving in random straight line motion.
D)The volume of the particles is negligible compared to the volume of the gas.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
71
Each of three identical 15.0-L gas cylinders contains 7.50 mol of gas at 295 K.Cylinder A contains Ar,cylinder B contains Cl2,and cylinder C contains N2.According to the kinetic molecular theory,which gas has the highest collision frequency?
A)Ar
B)Cl2
C)N2
D)All have identical collision frequencies
A)Ar
B)Cl2
C)N2
D)All have identical collision frequencies

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
72
At what temperature will sulfur hexafluoride molecules have the same average speed as argon atoms at 20°C?
A)-22.0°C
B)73.2°C
C)381°C
D)799°C
A)-22.0°C
B)73.2°C
C)381°C
D)799°C

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
73
Each of three identical 15.0-L gas cylinders contains 7.50 mol of gas at 295 K.Cylinder A contains Ar,cylinder B contains Cl2,and cylinder C contains N2.According to the kinetic molecular theory,which gas has the highest average kinetic energy?
A)Ar
B)Cl2
C)N2
D)All have identical average kinetic energies
A)Ar
B)Cl2
C)N2
D)All have identical average kinetic energies

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
74
You are given two flasks of equal volume.One contains H2 at 0°C and 1 atm while the other contains CO2 at 0°C and 2 atm.Which of the following quantities will be the same for both flasks?
A)average molecular kinetic energy
B)average molecular speed
C)density
D)number of molecules present
A)average molecular kinetic energy
B)average molecular speed
C)density
D)number of molecules present

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
75
Some assumptions from the kinetic molecular theory are listed below.Which one is most frequently cited to explain Charles' law?
A)The average kinetic energy of gas particles is proportional to the Kelvin temperature.
B)Collisions of gas particles are elastic and total kinetic energy of the gas is constant.
C)A gas consists of tiny particles moving in random straight line motion.
D)The volume of the particles is negligible compared to the volume of the gas.
A)The average kinetic energy of gas particles is proportional to the Kelvin temperature.
B)Collisions of gas particles are elastic and total kinetic energy of the gas is constant.
C)A gas consists of tiny particles moving in random straight line motion.
D)The volume of the particles is negligible compared to the volume of the gas.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
76
A process by which gas molecules escape through a tiny hole in a membrane into a vacuum without collisions is called
A)Boyle's law.
B)diffusion.
C)effusion.
D)sublimation.
A)Boyle's law.
B)diffusion.
C)effusion.
D)sublimation.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
77
Which one of the following gases will have the highest rate of effusion?
A)NO2
B)N2O
C)N2O4
D)NO3
A)NO2
B)N2O
C)N2O4
D)NO3

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
78
Some assumptions from the kinetic molecular theory are listed below.Which one is most frequently cited to explain compressibility of a gas?
A)The average kinetic energy of gas particles is proportional to the Kelvin temperature.
B)Collisions of gas particles are elastic and total kinetic energy of the gas is constant.
C)A gas consist of tiny particles moving in random straight line motion.
D)The volume of the particles is negligible compared to the volume of the gas.
A)The average kinetic energy of gas particles is proportional to the Kelvin temperature.
B)Collisions of gas particles are elastic and total kinetic energy of the gas is constant.
C)A gas consist of tiny particles moving in random straight line motion.
D)The volume of the particles is negligible compared to the volume of the gas.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following gases has the lowest average speed at 25°C?
A)CH4
B)H2S
C)NH3
D)O2
A)CH4
B)H2S
C)NH3
D)O2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following gases has the highest average speed at 400K?
A)CO2
B)N2O4
C)SF6
D)UF6
A)CO2
B)N2O4
C)SF6
D)UF6

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck


