Exam 9: Gases: Their Properties and Behavior
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
The principal cause of man-made ozone depletion in the stratosphere is due to
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
D
Some assumptions from the kinetic molecular theory are listed below.Which one is most frequently cited to explain compressibility of a gas?
Free
(Multiple Choice)
4.8/5  (25)
(25)
Correct Answer:
D
A 75.0 L steel tank at 20.0°C contains acetylene gas,C2H2,at a pressure of 1.39 atm.Assuming ideal behavior,how many grams of acetylene are in the tank?
Free
(Multiple Choice)
4.7/5  (32)
(32)
Correct Answer:
C
If CO2 and NH3 are allowed to effuse through a porous membrane under identical conditions,the rate of effusion for NH3 will be ________ times that of CO2.
(Multiple Choice)
4.8/5  (38)
(38)
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1).The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2)-(4)most closely represents the result of doubling the pressure and number of moles of gas while keeping the temperature constant? 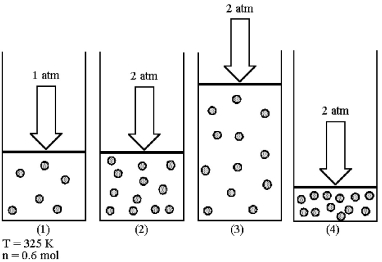
(Multiple Choice)
4.8/5  (37)
(37)
In the laboratory,hydrogen gas is usually made by the following reaction:
Zn(s)+ 2 HCl(aq)→ H2(g)+ ZnCl2(aq)
How many liters of H2 gas,collected over water at an atmospheric pressure of 752 mm Hg and a temperature of 21.0°C,can be made from 3.566 g of Zn and excess HCl? The partial pressure of water vapor is 18.65 mm Hg at 21.0°C.
(Multiple Choice)
4.7/5  (44)
(44)
If the pressure in a gas container that is connected to an open-end U-tube manometer is 106 kPa and the pressure of the atmosphere at the open end of the tube is 742 mm Hg,the level of mercury in the tube will be
(Multiple Choice)
4.9/5  (36)
(36)
In the diagram below,helium atoms are represented by unshaded spheres,neon atoms by gray spheres,and argon atoms by black spheres.  -If the total pressure in the container is 900 mm Hg,what is the partial pressure of argon?
-If the total pressure in the container is 900 mm Hg,what is the partial pressure of argon?
(Multiple Choice)
4.9/5  (40)
(40)
If 1.0 mol of N2 and 3.0 mol of H2 in a closed container initially at STP react completely in the reaction shown below,then the final pressure in the flask will be ________ atm at 273 K.
N2(g)+ 3 H2(g)→ 2 NH3(g)
(Short Answer)
4.8/5  (35)
(35)
Suppose you needed to closely monitor small changes in pressure inside a container using an open end manometer.For the best accuracy,the substance in the manometer should
(Multiple Choice)
4.8/5  (32)
(32)
Assume that you have a mixture of nitrogen (molecular mass = 28 amu),represented by unshaded spheres,and chlorine (molecular mass = 71 amu),represented by shaded spheres at 300 K.Which of the drawings best represents the mixture? 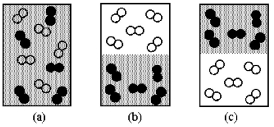
(Multiple Choice)
4.9/5  (40)
(40)
A gas bottle contains 0.250 mol of gas at 730 mm Hg pressure.If the final pressure is 1.15 atm,how many moles of gas were added to the bottle?
(Multiple Choice)
4.8/5  (47)
(47)
A basketball is inflated to a pressure of 1.50 atm in a 20.0°C garage.What is the pressure of the basketball outside where the temperature is -5.00°C?
(Multiple Choice)
4.8/5  (38)
(38)
Each of three identical 15.0-L gas cylinders contains 7.50 mol of gas at 295 K.Cylinder A contains Ar,cylinder B contains Cl2,and cylinder C contains N2.According to the kinetic molecular theory,which gas has the highest density?
(Multiple Choice)
4.8/5  (41)
(41)
When 15.0 g of zinc metal reacts with excess HCl,how many liters of H2 gas are produced at STP?
(Multiple Choice)
4.9/5  (46)
(46)
The apparatus shown is called a closed-end manometer because the arm not connected to the gas sample is closed to the atmosphere and is under vacuum. 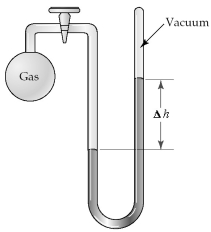 -What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 745 mm Hg and the difference in mercury levels,Δh,is 25 mm Hg?
-What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 745 mm Hg and the difference in mercury levels,Δh,is 25 mm Hg?
(Multiple Choice)
4.8/5  (31)
(31)
The molecule believed to be most responsible for global warming is ________.
(Short Answer)
4.9/5  (33)
(33)
What is the temperature of CO2 gas if the average speed (actually the root-mean-square speed)of the molecules is 750 m/s?
(Multiple Choice)
4.8/5  (45)
(45)
Showing 1 - 20 of 182
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)