Deck 3: Calculations With Chemical Formulas and Equations
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/139
Play
Full screen (f)
Deck 3: Calculations With Chemical Formulas and Equations
1
What is the molar mass of ammonium sulfite,(NH4)2SO3?
A)98 g/mol
B)116 g/mol
C)55 g/mol
D)180 g/mol
E)84 g/mol
A)98 g/mol
B)116 g/mol
C)55 g/mol
D)180 g/mol
E)84 g/mol
116 g/mol
2
How many atoms of carbon are there in 0.37 mol of procaine,C13H20N2O2,a "pain killer" used by dentists?
A)4.8 × 1023
B)3.1 × 1024
C)2.9 × 1024
D)4.5 × 1023
E)3.3 × 1024
A)4.8 × 1023
B)3.1 × 1024
C)2.9 × 1024
D)4.5 × 1023
E)3.3 × 1024
2.9 × 1024
3
Which of the following compounds contains the largest number of atoms?
A)4.0 mol of H2S
B)3.0 mol of NO3
C)2.0 mol of Na2SO4
D)5.0 mol of HBr
E)1.0 mol of CH3COOH
A)4.0 mol of H2S
B)3.0 mol of NO3
C)2.0 mol of Na2SO4
D)5.0 mol of HBr
E)1.0 mol of CH3COOH
2.0 mol of Na2SO4
4
Monodisperse polyacrylonitrile contains molecules with the general formula -(CH2CHCN)n-,where n is typically greater than 10,000.Given that a sample of monodisperse polyacrilonitrile weighs 676.8 g and contains 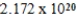 molecules of -(CH2CHCN)n-,calculate n.
molecules of -(CH2CHCN)n-,calculate n.
A)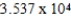
B)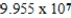
C)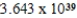
D)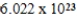
E)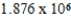
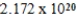 molecules of -(CH2CHCN)n-,calculate n.
molecules of -(CH2CHCN)n-,calculate n.A)
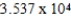
B)
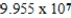
C)
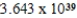
D)
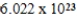
E)
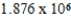

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
5
What is the formula mass of barium phosphate,Ba3(PO4)2?
A)506.95 amu
B)570.95 amu
C)232.30 amu
D)1013.90 amu
E)601.92 amu
A)506.95 amu
B)570.95 amu
C)232.30 amu
D)1013.90 amu
E)601.92 amu

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
6
A 1.74 g sample of an element contains 7.887 ×1021atoms.What is the element symbol?
A)Cs
B)I
C)In
D)Sb
E)Cd
A)Cs
B)I
C)In
D)Sb
E)Cd

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
7
Plastic wrap can be made from poly(vinylidene chloride).A single poly(vinylidene chloride)strand has the general formula -(CH2CHCl)n-,where n ranges from 10,000 to 100,000.What is the molar mass of a single poly(vinylidene chloride)molecule containing 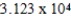 repeating units?
repeating units?
A)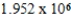 g/mol
g/mol
B)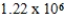 g/mol
g/mol
C)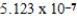 g/mol
g/mol
D)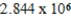 g/mol
g/mol
E)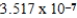 g/mol
g/mol
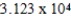 repeating units?
repeating units?A)
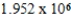 g/mol
g/molB)
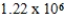 g/mol
g/molC)
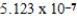 g/mol
g/molD)
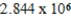 g/mol
g/molE)
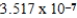 g/mol
g/mol
Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
8
The formula mass of aluminum oxalate,Al2(C2O4)3,is
A)143 amu.
B)318 amu.
C)290 amu.
D)204 amu.
E)197 amu.
A)143 amu.
B)318 amu.
C)290 amu.
D)204 amu.
E)197 amu.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
9
What is the molecular mass of cycloheptane,C7H14?
A)13.02 amu
B)1191.19 amu
C)85.08 amu
D)98.19 amu
E)26.12 amu
A)13.02 amu
B)1191.19 amu
C)85.08 amu
D)98.19 amu
E)26.12 amu

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
10
The dicarboxylic acid potassium hydrogen pthalate (shown in the figure)is used to standardize solutions of strong base.What is the molar mass of this compound? 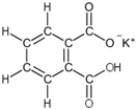
A)204.2 g/mol
B)192.2 g/mol
C)248.9 g/mol
D)71.08 g/mol
E)172.2 g/mol

A)204.2 g/mol
B)192.2 g/mol
C)248.9 g/mol
D)71.08 g/mol
E)172.2 g/mol

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
11
The formula mass of zinc acetate trihydrate,Zn(CH3COO)2 • 3H2O,is
A)356 amu.
B)184 amu.
C)292 amu.
D)238 amu.
E)156 amu.
A)356 amu.
B)184 amu.
C)292 amu.
D)238 amu.
E)156 amu.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
12
The hydrocarbon octane has the structural formula CH3(CH2)6CH3.What is the molecular mass of this hydrocarbon?
A)114.2 amu.
B)0.008754 amu.
C)100.1 amu.
D)0.009984432 amu.
E)124.2 amu.
A)114.2 amu.
B)0.008754 amu.
C)100.1 amu.
D)0.009984432 amu.
E)124.2 amu.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
13
What is the molecular mass of the hydrocarbon styrene (shown in the figure)? 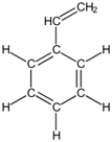
A)104.1 amu.
B)91.1 amu.
C)103.1 amu.
D)13.0 amu.
E)78.1 amu.

A)104.1 amu.
B)91.1 amu.
C)103.1 amu.
D)13.0 amu.
E)78.1 amu.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
14
What is the mass in grams of one propene,C3H6,molecule?
A)6.99× 10-23 g
B)2.53 × 1025 g
C)44.0 g
D)42.0 g
E)1.99 × 10-23 g
A)6.99× 10-23 g
B)2.53 × 1025 g
C)44.0 g
D)42.0 g
E)1.99 × 10-23 g

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
15
The fully hydrated form of sodium sulfate is the decahydrate,Na2SO4 • 10H2O.When heated the hydrated salt loses water.How many water molecules are found per formula unit in a partially dehydrated sample of sodium sulfate with a formula mass of 197 amu (i.e.find n for Na2SO4 • nH2O)?
A)3 waters.
B)5 waters.
C)7 waters.
D)9 waters.
E)4 waters.
A)3 waters.
B)5 waters.
C)7 waters.
D)9 waters.
E)4 waters.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
16
The molecular formula of a particular solid is C8H4O6.Its molecular mass is
A)288 amu.
B)100 amu.
C)296 amu.
D)196 amu.
E)170 amu.
A)288 amu.
B)100 amu.
C)296 amu.
D)196 amu.
E)170 amu.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
17
What is the mass of carbon in grams found in one molecule of the compound C7H8O4?
A)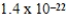 g
g
B)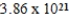 g
g
C)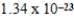 g
g
D)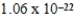 g
g
E)156.000000 g
A)
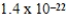 g
gB)
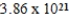 g
gC)
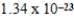 g
gD)
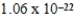 g
gE)156.000000 g

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
18
A single molecule of polystryene has the repeating unit -[CH2CH(C6H5)]n-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is ![<strong>A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?</strong> A) units. B) units. C) units. D) units. E) units.](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_30dd_a82d_25d058ee1e7a_TB2288_11.jpg) amu?
amu?
A)![<strong>A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?</strong> A) units. B) units. C) units. D) units. E) units.](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_30de_a82d_7ff72cb8130f_TB2288_11.jpg) units.
units.
B)![<strong>A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?</strong> A) units. B) units. C) units. D) units. E) units.](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_57ef_a82d_77944ba92449_TB2288_11.jpg) units.
units.
C)![<strong>A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?</strong> A) units. B) units. C) units. D) units. E) units.](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_57f0_a82d_dba899a22772_TB2288_11.jpg) units.
units.
D)![<strong>A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?</strong> A) units. B) units. C) units. D) units. E) units.](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_57f1_a82d_915bd108cf04_TB2288_11.jpg) units.
units.
E)![<strong>A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?</strong> A) units. B) units. C) units. D) units. E) units.](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_57f2_a82d_97c5135cc652_TB2288_11.jpg) units.
units.
![<strong>A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?</strong> A) units. B) units. C) units. D) units. E) units.](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_30dd_a82d_25d058ee1e7a_TB2288_11.jpg) amu?
amu?A)
![<strong>A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?</strong> A) units. B) units. C) units. D) units. E) units.](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_30de_a82d_7ff72cb8130f_TB2288_11.jpg) units.
units.B)
![<strong>A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?</strong> A) units. B) units. C) units. D) units. E) units.](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_57ef_a82d_77944ba92449_TB2288_11.jpg) units.
units.C)
![<strong>A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?</strong> A) units. B) units. C) units. D) units. E) units.](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_57f0_a82d_dba899a22772_TB2288_11.jpg) units.
units.D)
![<strong>A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?</strong> A) units. B) units. C) units. D) units. E) units.](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_57f1_a82d_915bd108cf04_TB2288_11.jpg) units.
units.E)
![<strong>A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?</strong> A) units. B) units. C) units. D) units. E) units.](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_57f2_a82d_97c5135cc652_TB2288_11.jpg) units.
units.
Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
19
Monodisperse polyacrylonitrile contains molecules with the general formula -(CH2CHCN)n-,where n is typically greater than 10,000.Given that a sample of monodisperse polyacrylonitrile weighs 665.1 g and contains 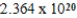 molecules of -(CH2CHCN)n-,what is the molar mass of the polymer?
molecules of -(CH2CHCN)n-,what is the molar mass of the polymer?
A)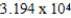 g/mol
g/mol
B)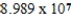 g/mol
g/mol
C)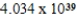 g/mol
g/mol
D)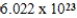 g/mol
g/mol
E)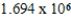 g/mol
g/mol
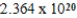 molecules of -(CH2CHCN)n-,what is the molar mass of the polymer?
molecules of -(CH2CHCN)n-,what is the molar mass of the polymer?A)
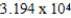 g/mol
g/molB)
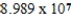 g/mol
g/molC)
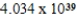 g/mol
g/molD)
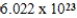 g/mol
g/molE)
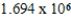 g/mol
g/mol
Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
20
What is the molar mass of the solid C6H8N2O4?
A)144 g/mol
B)108 g/mol
C)172 g/mol
D)90 g/mol
E)164 g/mol
A)144 g/mol
B)108 g/mol
C)172 g/mol
D)90 g/mol
E)164 g/mol

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
21
What is the mass of oxygen atoms in 0.380 mol Fe(CO)5?
A)21.2 g
B)74.4 g
C)30.4 g
D)6.08 g
E)22.8 g
A)21.2 g
B)74.4 g
C)30.4 g
D)6.08 g
E)22.8 g

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
22
How many moles of silver are contained in 7.00 kg of silver?
A)64.9 mol
B)64.9 × 101 mol
C)64.9 × 10-3 mol
D)64.9 × 103 mol
E)64.9 × 10-1 mol
A)64.9 mol
B)64.9 × 101 mol
C)64.9 × 10-3 mol
D)64.9 × 103 mol
E)64.9 × 10-1 mol

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
23
How many molecules are there in 1.54 kg of hydrazine,N2H4?
A)4.81 × 1023
B)2.90 × 1025
C)1.88 × 1022
D)1.54 × 1026
E)2.56 × 1021
A)4.81 × 1023
B)2.90 × 1025
C)1.88 × 1022
D)1.54 × 1026
E)2.56 × 1021

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
24
What is the mass in grams of 0.754 mol of glucose,C6H12O6?
A)0.00419 g
B)72.4 g
C)136 g
D)22.6 g
E)239 g
A)0.00419 g
B)72.4 g
C)136 g
D)22.6 g
E)239 g

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following contains the greatest mass of oxygen atoms?
A)0.5 mol CoSO4 • 7H2O
B)2.1 mol KHSO4
C)1.1 mol K2Cr2O7
D)2.1 mol H2O2
E)2.1 mol Na2S2O3
A)0.5 mol CoSO4 • 7H2O
B)2.1 mol KHSO4
C)1.1 mol K2Cr2O7
D)2.1 mol H2O2
E)2.1 mol Na2S2O3

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
26
Sodium cyclamate,C6H11NHSO3Na,was used at one time as an artificial sweetener.C6H11NHSO3Na has a molecular mass of 201.2 g/mol.How many moles of sodium cyclamate are contained in a 56.0-g sample?
A)0.175 mol
B)0.160 mol
C)0.278 mol
D)0.255 mol
E)%1,0f mol
A)0.175 mol
B)0.160 mol
C)0.278 mol
D)0.255 mol
E)%1,0f mol

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
27
How many moles of pentane,C5H12,are contained in a 31-g sample?
A)0.52 mol
B)0.43 mol
C)0.74 mol
D)3.9 mol
E)3.1 mol
A)0.52 mol
B)0.43 mol
C)0.74 mol
D)3.9 mol
E)3.1 mol

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate the number of moles of bromine present in 20.5 mL of Br2(l),whose density is 3.12 g/mL.
A)2.50 mol
B)0.257 mol
C)0.801 mol
D)0.128 mol
E)0.400 mol
A)2.50 mol
B)0.257 mol
C)0.801 mol
D)0.128 mol
E)0.400 mol

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
29
Sorbose,C6H12O6,is used in making vitamin C.A sorbose sample containing 36.0 g of carbon atoms also contains ____ g of hydrogen atoms.
A)12
B)6.04
C)4.32 × 102
D)36
E)2.99
A)12
B)6.04
C)4.32 × 102
D)36
E)2.99

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
30
A 0.0106-mol sample of urea,NH2CONH2,contains
A)6.02 × 1023 molecules.
B)2.55 × 1022 molecules.
C)5.10 × 1022 atoms.
D)2.55 × 1023 atoms.
E)1.06 × 1024 atoms.
A)6.02 × 1023 molecules.
B)2.55 × 1022 molecules.
C)5.10 × 1022 atoms.
D)2.55 × 1023 atoms.
E)1.06 × 1024 atoms.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
31
How many molecules are there in 104 g of pentylene glycol,HO(CH2)5OH?
A)1
B)(6.02 × 1023)/104
C)104
D)104 × (6.02 × 1023)
E)6.02 × 1023
A)1
B)(6.02 × 1023)/104
C)104
D)104 × (6.02 × 1023)
E)6.02 × 1023

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the following three samples.
A.A sample containing 180 g glucose (C6H12O6)
B.A sample containing 90 g glucose and 90 g mannose (C6H12O6)
C.A sample containing 180 g mannose
Which statement is correct?
A)All three samples have the same number of oxygen atoms.
B)Both samples A and C have the same number of oxygen atoms,but more than in sample B.
C)Sample B has more oxygen atoms than sample A or sample C.
D)Sample C has more oxygen atoms than sample A or sample B
E)Sample A has more oxygen atoms than sample B or sample C.
A.A sample containing 180 g glucose (C6H12O6)
B.A sample containing 90 g glucose and 90 g mannose (C6H12O6)
C.A sample containing 180 g mannose
Which statement is correct?
A)All three samples have the same number of oxygen atoms.
B)Both samples A and C have the same number of oxygen atoms,but more than in sample B.
C)Sample B has more oxygen atoms than sample A or sample C.
D)Sample C has more oxygen atoms than sample A or sample B
E)Sample A has more oxygen atoms than sample B or sample C.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
33
How many moles of hexachlorobenzene,C6Cl6,are in 5.44 g of C6Cl6?
A)0.0254 mol
B)1.55 × 103 mol
C)0.0755 mol
D)0.019 mol
E)0.0394 mol
A)0.0254 mol
B)1.55 × 103 mol
C)0.0755 mol
D)0.019 mol
E)0.0394 mol

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
34
In 0.266 mol of trimellitic acid,C6H3(COOH)3,there are
A)1.60 × 1022 molecules.
B)6.41 × 1024 molecules.
C)4.80 × 1023 oxygen atoms.
D)1.44 × 1024 carbon atoms.
E)2.67 × 1023 hydrogen atoms.
A)1.60 × 1022 molecules.
B)6.41 × 1024 molecules.
C)4.80 × 1023 oxygen atoms.
D)1.44 × 1024 carbon atoms.
E)2.67 × 1023 hydrogen atoms.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
35
How many molecules are there in 60 g of acetic acid,C2H4O2?
A)(6.02 × 1023)/ 60
B)60
C)6.02 × 1023
D)30
E)60 × (6.02 × 1023)
A)(6.02 × 1023)/ 60
B)60
C)6.02 × 1023
D)30
E)60 × (6.02 × 1023)

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
36
How many moles of iron atoms are contained in 4.16 g of iron?
A)232 mol
B)0.0548 mol
C)0.0745 mol
D)0.116 mol
E)0.160 mol
A)232 mol
B)0.0548 mol
C)0.0745 mol
D)0.116 mol
E)0.160 mol

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
37
A sample of ammonium phosphate,(NH4)3PO4,contains 0.104 mol of nitrogen atoms.The number of moles of oxygen atoms in the sample is
A)0.139 mol.
B)0.0104 mol.
C)0.41 mol.
D)0.419 mol.
E)4.6 mol.
A)0.139 mol.
B)0.0104 mol.
C)0.41 mol.
D)0.419 mol.
E)4.6 mol.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
38
A sample of thallium(III)peroxide,Tl2(O2)3,contains 2.45 mol of thallium(III)ions.The number of moles of peroxide ions in the sample is
A)3.67 mol.
B)1.63 mol.
C)2.45 mol.
D)7.35 mol.
E)4.9361 mol.
A)3.67 mol.
B)1.63 mol.
C)2.45 mol.
D)7.35 mol.
E)4.9361 mol.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
39
Which one of the following samples has the greatest mass?
A)0.37 mol of camphor,C10H16O
B)4.2 mol of ammonia,NH3
C)9.3 mol of krypton,Kr
D)4.0 mol of iodine vapor,I2
E)1.6 mol of formaldehyde,CH2O
A)0.37 mol of camphor,C10H16O
B)4.2 mol of ammonia,NH3
C)9.3 mol of krypton,Kr
D)4.0 mol of iodine vapor,I2
E)1.6 mol of formaldehyde,CH2O

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
40
Styrene's empirical formula is CH.What mass of styrene contains 2.95 × 1021 atoms of hydrogen? The molar mass of styrene is 104 g/mol.
A)0.0391 g
B)0.0636 g
C)0.509 g
D)0.0587 g
E)0.00489 g
A)0.0391 g
B)0.0636 g
C)0.509 g
D)0.0587 g
E)0.00489 g

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
41
An ore sample with a mass of 92.3 g is found to contain 17.7% by mass iron.What mass of iron is contained in the ore?
A)16.300000 g
B)78.4 g
C)1.77 g
D)82.2 g
E)464 g
A)16.300000 g
B)78.4 g
C)1.77 g
D)82.2 g
E)464 g

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following samples contains the smallest number of molecules?
A)5.00 g of TNT,C7H5N3O6
B)5.00 g of benzene,C6H6
C)5.00 g of glucose,C6H12O6
D)5.00 g of naphthalene,C10H8
E)5.00 g of formaldehyde,CH2O
A)5.00 g of TNT,C7H5N3O6
B)5.00 g of benzene,C6H6
C)5.00 g of glucose,C6H12O6
D)5.00 g of naphthalene,C10H8
E)5.00 g of formaldehyde,CH2O

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
43
The mineral leadhillite,which is essentially Pb4(SO4)(CO3)2(OH)2 (FW = 1079 g/mol),contains ____% hydrogen by mass.
A)76.81
B)0.1868
C)2.226
D)17.79
E)2.971
A)76.81
B)0.1868
C)2.226
D)17.79
E)2.971

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following samples contains the largest number of atoms?
A)1 g N2
B)1 g Be
C)1 g Br2
D)1 g P4
E)1 g Si
A)1 g N2
B)1 g Be
C)1 g Br2
D)1 g P4
E)1 g Si

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
45
How many molecules are there in 2.10 mg of mannose,C6H12O6,which is a sweet-tasting sugar that has a bitter aftertaste?
A)5.16 × 1021
B)2.87 × 1021
C)6.32 × 1018
D)3.49 × 1024
E)7.02 × 1018
A)5.16 × 1021
B)2.87 × 1021
C)6.32 × 1018
D)3.49 × 1024
E)7.02 × 1018

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
46
The total number of oxygen atoms in 1.93 g of CaCO3 (MM = 100.0 g/mol)is
A)2.24 × 1023.
B)4.65 × 1022.
C)3.49 × 1022.
D)1.92 × 1023.
E)5.81 × 1022.
A)2.24 × 1023.
B)4.65 × 1022.
C)3.49 × 1022.
D)1.92 × 1023.
E)5.81 × 1022.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
47
What is the percentage by mass of chlorine in the insecticide Lindane,C6H6Cl6?
A)20.6 %
B)1.00 %
C)69.6 %
D)8.80 %
E)73.1 %
A)20.6 %
B)1.00 %
C)69.6 %
D)8.80 %
E)73.1 %

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
48
Which one of the following contains 4.21 × 1024 atoms?
A)91 g C2H2
B)168 g O2
C)56 g CH4
D)294 g N2
E)28 g He
A)91 g C2H2
B)168 g O2
C)56 g CH4
D)294 g N2
E)28 g He

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
49
In 0.300 mol of dimethylhydrazine,(CH3)2N2H2,there are
A)1.81 × 1024 molecules.
B)2.51 × 1023 atoms.
C)1.81 × 1022 molecules.
D)2.17 × 1024 atoms.
E)1.08 × 1024 atoms.
A)1.81 × 1024 molecules.
B)2.51 × 1023 atoms.
C)1.81 × 1022 molecules.
D)2.17 × 1024 atoms.
E)1.08 × 1024 atoms.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
50
An ore sample is found to contain 10.500000 g of cobalt and 87.3 g waste rock (gangue).What is the percent by mass of cobalt in the ore?
A)10.7 %
B)12.114 %
C)0.107 %
D)0.12228 %
E)1.2879 %
A)10.7 %
B)12.114 %
C)0.107 %
D)0.12228 %
E)1.2879 %

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
51
A sample of 496 g of white phosphorus,P4,contains the same number of atoms as
A)192 g of ozone (O3).
B)56 g of nitrogen (N2).
C)92 g of sodium.
D)120 g of formaldehyde (CH2O).
E)128 g of oxygen (O2).
A)192 g of ozone (O3).
B)56 g of nitrogen (N2).
C)92 g of sodium.
D)120 g of formaldehyde (CH2O).
E)128 g of oxygen (O2).

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
52
How many atoms are present in 495 g of KPF6 (MM = 184.1 g/mol)?
A)2.38 × 1025
B)1.62 × 1021
C)2.69 × 1021
D)1.21 × 1026
E)1.29 × 1025
A)2.38 × 1025
B)1.62 × 1021
C)2.69 × 1021
D)1.21 × 1026
E)1.29 × 1025

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
53
Styrene's empirical formula is CH.When it is heated to 200°C,it is converted into a polymer,polystyrene,which has excellent insulating properties.What mass of styrene contains 1.77 × 1021 molecules of styrene? The molar mass of styrene is 104 g/mol.
A)0.00293 g
B)0.305 g
C)0.587 g
D)0.0382 g
E)0.0235 g
A)0.00293 g
B)0.305 g
C)0.587 g
D)0.0382 g
E)0.0235 g

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
54
Which is a reasonable mass corresponding to 1026 molecules of a substance?
A)100 ng
B)100 g
C)100 mg
D)100 kg
E)100 µg
A)100 ng
B)100 g
C)100 mg
D)100 kg
E)100 µg

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following compounds has the highest percentage of hydrogen atoms by mass?
A)CH3COOH
B)C2H5OH
C)CH3OH
D)H2CO3
E)H2C2O4
A)CH3COOH
B)C2H5OH
C)CH3OH
D)H2CO3
E)H2C2O4

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
56
How many aluminum atoms are there in 52 g of Al2S3?
A)4.2 × 1023
B)1.6 × 1021
C)2.1 × 1023
D)1.1 × 1021
E)6.3 × 1023
A)4.2 × 1023
B)1.6 × 1021
C)2.1 × 1023
D)1.1 × 1021
E)6.3 × 1023

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
57
A sample of 96 g of ozone,O3,contains the same number of atoms as
A)96 g of oxygen (O2).
B)8.06 g of hydrogen (H2).
C)76 g of fluorine (F2).
D)54 g of aluminum (Al).
E)117 g of nickel (Ni).
A)96 g of oxygen (O2).
B)8.06 g of hydrogen (H2).
C)76 g of fluorine (F2).
D)54 g of aluminum (Al).
E)117 g of nickel (Ni).

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
58
What is the percent by mass nitrogen in (NH4)2SO3?
A)24.1 %
B)12.961 %
C)64.6 %
D)39.3 %
E)4.73 %
A)24.1 %
B)12.961 %
C)64.6 %
D)39.3 %
E)4.73 %

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following compounds has the highest percentage of nitrogen by mass?
A)(NH4)2SO3
B)NaNO3
C)N2F4
D)NH4NO2
E)HNO3
A)(NH4)2SO3
B)NaNO3
C)N2F4
D)NH4NO2
E)HNO3

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
60
In 1928,1.0 g of rhenium,Re,was isolated from 660 kg of the ore molybenite.The percent by mass of this element in the molybenite was
A)0.66 %.
B)0.15 %.
C)3.5 × 10-4 %.
D)6.6 × 10-3 %.
E)1.5 × 10-4 %.
A)0.66 %.
B)0.15 %.
C)3.5 × 10-4 %.
D)6.6 × 10-3 %.
E)1.5 × 10-4 %.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is the empirical formula for the molecule below? 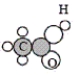
A)CHO
B)CH3COOH
C)C2H4O2
D)CH2O
E)none of the above.

A)CHO
B)CH3COOH
C)C2H4O2
D)CH2O
E)none of the above.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
62
Analysis of a compound showed that it contained 76.0 % fluorine atoms and 24.0 % carbon atoms by mass.What is its empirical formula?
A)CF2
B)C2F3
C)CF3
D)C2F5
E)CF
A)CF2
B)C2F3
C)CF3
D)C2F5
E)CF

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
63
A sample of an oxide of antimony (Sb)contained 48.5 g of antimony combined with 15.9 g of oxygen.What is the simplest formula for the oxide?
A)SbO2
B)SbO
C)Sb2O3
D)Sb3O
E)Sb2O5
A)SbO2
B)SbO
C)Sb2O3
D)Sb3O
E)Sb2O5

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
64
A 4.215 g sample of a compound containing only carbon,hydrogen,and oxygen is burned in an excess of dioxygen,producing 9.582 g CO2 and 3.922 g H2O.What percent by mass of oxygen is contained in the original sample?
A)27.54 %
B)32.75 %
C)12.73 %
D)13.42 %
E)6.939 %
A)27.54 %
B)32.75 %
C)12.73 %
D)13.42 %
E)6.939 %

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
65
NaHCO3 is the active ingredient in baking soda.How many grams of oxygen are present in 0.25 g of NaHCO3?
A)0.009 g
B)0.048 g
C)2.98 × 103 g
D)0.016 g
E)0.14g
A)0.009 g
B)0.048 g
C)2.98 × 103 g
D)0.016 g
E)0.14g

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
66
Of the following,the only empirical formula is
A)C4H10.
B)C4H8.
C)C5H14.
D)H2O2.
E)O3.
A)C4H10.
B)C4H8.
C)C5H14.
D)H2O2.
E)O3.

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following contains the greatest mass of bromine atoms?
A)15.0 g of KBr
B)29.0 g of Br2
C)0.096 mol of KBr
D)0.078 mol of Br2
E)25.0 g of NaBrO3
A)15.0 g of KBr
B)29.0 g of Br2
C)0.096 mol of KBr
D)0.078 mol of Br2
E)25.0 g of NaBrO3

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
68
How many grams of potassium are present in 28.7 g of K2Cr2O7?
A)7.63 g
B)1.468 g
C)3.81 g
D)78.2 g
E)14.4 g
A)7.63 g
B)1.468 g
C)3.81 g
D)78.2 g
E)14.4 g

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
69
A 3.391 g sample of a compound containing only carbon,hydrogen,and oxygen is burned in an excess of dioxygen,producing 6.477 g CO2 and 3.978 g H2O.What mass of oxygen is contained in the original sample?
A)1.178 g
B)1.4899 g
C)3.086 g
D)2.499 g
E)0.5874 g
A)1.178 g
B)1.4899 g
C)3.086 g
D)2.499 g
E)0.5874 g

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
70
What is the mass percentage of carbon in the compound C6H6O3?
A)4.8 %
B)61.9 %
C)57.1 %
D)38.1 %
E)20.0 %
A)4.8 %
B)61.9 %
C)57.1 %
D)38.1 %
E)20.0 %

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
71
A 4.957 g sample of a hydrocarbon is burned in an excess of dioxygen,producing 9.284 g CO2 and water.What mass of hydrogen is contained in the original sample?
A)2.423 g
B)4.327 g
C)14.24 g
D)1.794 g
E)2.478 g
A)2.423 g
B)4.327 g
C)14.24 g
D)1.794 g
E)2.478 g

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
72
A compound containing only carbon,hydrogen,and oxygen is subjected to elemental analysis.Upon complete combustion,a 0.7916-g sample of the compound produced 1.581 g of CO2 and 0.6474 g of H2O.What is the empirical formula of the compound?
A)C3H6O3
B)C3H3O
C)C2H4O
D)C2H2O
E)CH2O3
A)C3H6O3
B)C3H3O
C)C2H4O
D)C2H2O
E)CH2O3

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following compounds has the same percentage of carbon and hydrogen by mass as cyclohexane,C6H12?
A)C6H14,hexane
B)C4H8,butylene
C)C6H10,cyclohexene
D)C6H6,benzene
E)C6H12O6,glucose
A)C6H14,hexane
B)C4H8,butylene
C)C6H10,cyclohexene
D)C6H6,benzene
E)C6H12O6,glucose

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
74
A sample containing only carbon,hydrogen,phosphorus,and oxygen is subjected to elemental analysis.After complete combustion,a 0.3584-g sample of the compound yields 0.5139 g of CO2,0.3155 g of H2O,and 0.2762 g of P4O10.What is the empirical formula of the compound?
A)CH3PO
B)C2H3PO
C)C2H6P2O4
D)C3H9PO
E)CH2P4O13
A)CH3PO
B)C2H3PO
C)C2H6P2O4
D)C3H9PO
E)CH2P4O13

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
75
A 3.903 g sample of a hydrocarbon is burned in an excess of dioxygen,producing 7.31177 g CO2 and 4.488 g H2O.What is the empirical formula of the hydrocarbon?
A)CH3
B)CH2
C)C2H3
D)CH4
E)CH
A)CH3
B)CH2
C)C2H3
D)CH4
E)CH

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
76
A crystal of the mineral troegerite,(UO2)3(AsO4)2 • 12H2O (FM = 1304 amu),contains ____% uranium by mass.
A)18.6
B)47.2
C)62.8
D)30.0
E)54.8
A)18.6
B)47.2
C)62.8
D)30.0
E)54.8

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
77
How many grams of hydrogen atoms are present in 16.4 g of water?
A)33.1 g
B)0.91 g
C)1.84 g
D)2.20 g
E)10.9 g
A)33.1 g
B)0.91 g
C)1.84 g
D)2.20 g
E)10.9 g

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
78
A sample containing only carbon,hydrogen,and silicon is subjected to elemental analysis.After complete combustion,a 0.7020-g sample of the compound yields 1.4 g of CO2,0.86 g of H2O,and 0.478 g of SiO2.What is the empirical formula of the compound?
A)CH3Si
B)C2H4Si
C)C4H12Si
D)C6H12Si2
E)CH2Si
A)CH3Si
B)C2H4Si
C)C4H12Si
D)C6H12Si2
E)CH2Si

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
79
A 0.4647-g sample of a compound known to contain only carbon,hydrogen,and oxygen was burned in dioxygen to yield 0.01962 mol of CO2 and 0.01961 mol of H2O.What is the empirical formula of the compound?
A)CHO
B)C3H3O2
C)C2H2O
D)C3H6O2
E)C6H3O2
A)CHO
B)C3H3O2
C)C2H2O
D)C3H6O2
E)C6H3O2

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck
80
What is the percentage by mass of nitrogen in ammonium phosphate,(NH4)3PO4?
A)28.1 %
B)14.15 %
C)52.4 %
D)39.9 %
E)2.27 %
A)28.1 %
B)14.15 %
C)52.4 %
D)39.9 %
E)2.27 %

Unlock Deck
Unlock for access to all 139 flashcards in this deck.
Unlock Deck
k this deck


