Exam 3: Calculations With Chemical Formulas and Equations
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
Which of the following is the empirical formula for the molecule below? 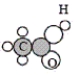
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
D
Of the following,the only empirical formula is
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
C
A crystal of the mineral troegerite,(UO2)3(AsO4)2 • 12H2O (FM = 1304 amu),contains ____% uranium by mass.
Free
(Multiple Choice)
4.7/5  (44)
(44)
Correct Answer:
E
The analysis of an organic compound showed that it contained 0.105 mol of C,0.315 mol of H,and 0.0350 mol of N.Its molecular mass is 59 amu.How many atoms of carbon are there in the empirical formula for the compound,and how many are in the molecular formula?
(Multiple Choice)
4.8/5  (43)
(43)
A 15-g sample of lithium is reacted with 15 g of fluorine to form lithium fluoride:
2Li + F2 → 2LiF.After the reaction is complete,what will be present?
(Multiple Choice)
4.8/5  (29)
(29)
How many atoms are present in 495 g of KPF6 (MM = 184.1 g/mol)?
(Multiple Choice)
4.7/5  (30)
(30)
The balanced equation for the combustion of ethanol is
2C2H5OH(g)+ 7O2(g)→ 4CO2(g)+ 6H2O(g)
How many grams of dioxygen are required to burn 3.4 g of C2H5OH?
(Multiple Choice)
4.9/5  (25)
(25)
An organic compound has a molar mass of 169.3 g/mol and contains 10.63 % hydrogen atoms by mass.How many hydrogen atoms are in each molecule of this compound?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following contains the greatest mass of bromine atoms?
(Multiple Choice)
4.7/5  (33)
(33)
What is the empirical formula of an oxide of nitrogen that contains 25.93 % nitrogen by mass?
(Multiple Choice)
4.8/5  (33)
(33)
If 49.0 g of O2 is mixed with 49.0 g of H2 and the mixture is ignited,what is the maximum mass of water that may be produced?
(Multiple Choice)
4.9/5  (27)
(27)
Chlorine was passed over 1.41 g of heated titanium,and 4.54 g of a chloride-containing compound of Ti was obtained.What is the empirical formula of the chloride-containing compound?
(Multiple Choice)
4.8/5  (29)
(29)
How many molecules are there in 2.10 mg of mannose,C6H12O6,which is a sweet-tasting sugar that has a bitter aftertaste?
(Multiple Choice)
4.8/5  (31)
(31)
When 20.0 g C2H6 and 60.0 g O2 react to form CO2 and H2O,how many grams of water are formed?
(Multiple Choice)
5.0/5  (34)
(34)
What is the percentage by mass of chlorine in the insecticide Lindane,C6H6Cl6?
(Multiple Choice)
5.0/5  (34)
(34)
The reaction of 11.9 g of CHCl3 with excess chlorine produced 11.0 g of CCl4,carbon tetrachloride:
2CHCl3 + 2Cl2 → 2CCl4 + 2HCl
What is the percent yield?
(Multiple Choice)
4.8/5  (28)
(28)
How many molecules are there in 60 g of acetic acid,C2H4O2?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 1 - 20 of 139
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)