Deck 6: Thermochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/119
Play
Full screen (f)
Deck 6: Thermochemistry
1
What is the change in internal energy of the system (ΔU)if 71 kJ of heat energy is absorbed by the system and 12 kJ of work is done on the system for a certain process?
A)83 kJ
B)59 kJ
C)71 kJ
D)-83 kJ
E)-59 kJ
A)83 kJ
B)59 kJ
C)71 kJ
D)-83 kJ
E)-59 kJ
83 kJ
2
Which of the following statements about enthalpy is false?
A)Enthalpy is a state function.
B)At constant pressure,the enthalpy change is equal to the heat absorbed or released.
C)Enthalpy is an extensive property.
D)The change in enthalpy of a process cannot be negative.
E)The SI unit of enthalpy is J.
A)Enthalpy is a state function.
B)At constant pressure,the enthalpy change is equal to the heat absorbed or released.
C)Enthalpy is an extensive property.
D)The change in enthalpy of a process cannot be negative.
E)The SI unit of enthalpy is J.
The change in enthalpy of a process cannot be negative.
3
If q = -96 kJ for a certain process,that process
A)requires a catalyst.
B)is exothermic.
C)occurs rapidly.
D)is endothermic.
E)cannot occur.
A)requires a catalyst.
B)is exothermic.
C)occurs rapidly.
D)is endothermic.
E)cannot occur.
is exothermic.
4
Which of the following is an endothermic process?
A)work is done by the system on the surroundings
B)heat energy flows from the system to the surroundings
C)work is done on the system by the surroundings
D)heat energy is evolved by the system
E)none of the above
A)work is done by the system on the surroundings
B)heat energy flows from the system to the surroundings
C)work is done on the system by the surroundings
D)heat energy is evolved by the system
E)none of the above

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
5
H2 and F2 react according to the following equation,forming HF.
H2(g)+ F2(g)→ 2HF(g); ΔH° = -271 kJ
If H2(g)and F2(g)were mixed in a thermally insulated vessel,the reaction that occurred would be
A)endothermic,and the temperature of the reaction system would fall.
B)We could not tell unless the original and final temperatures were given.
C)exothermic,and the temperature of the reaction system would fall.
D)exothermic,and the temperature of the reaction system would rise.
E)endothermic,and the temperature of the reaction system would rise.
H2(g)+ F2(g)→ 2HF(g); ΔH° = -271 kJ
If H2(g)and F2(g)were mixed in a thermally insulated vessel,the reaction that occurred would be
A)endothermic,and the temperature of the reaction system would fall.
B)We could not tell unless the original and final temperatures were given.
C)exothermic,and the temperature of the reaction system would fall.
D)exothermic,and the temperature of the reaction system would rise.
E)endothermic,and the temperature of the reaction system would rise.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements about heat is false?
A)If heat flows into a system,the extra energy of the system appears in the form of internal energy.
B)A hot object possesses more heat than a cold object.
C)If the system and surroundings are in thermal equilibrium,there is no heat flow between them.
D)A process in which heat flows out of a system is said to be exothermic.
E)Heat is a form of energy flow.
A)If heat flows into a system,the extra energy of the system appears in the form of internal energy.
B)A hot object possesses more heat than a cold object.
C)If the system and surroundings are in thermal equilibrium,there is no heat flow between them.
D)A process in which heat flows out of a system is said to be exothermic.
E)Heat is a form of energy flow.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements is incorrect?
A)Internal energy is a state function.
B)The value of q is positive when heat flows into a system from the surroundings.
C)The value of q is positive in an endothermic process.
D)Heat flows from a system into the surroundings in an endothermic process.
E)Enthalpy is a state function.
A)Internal energy is a state function.
B)The value of q is positive when heat flows into a system from the surroundings.
C)The value of q is positive in an endothermic process.
D)Heat flows from a system into the surroundings in an endothermic process.
E)Enthalpy is a state function.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements is true concerning the decomposition of liquid water to form hydrogen gas and oxygen gas?
2H2O(l)→ 2H2(g)+ O2(g)
A)ΔH is greater than ΔU because the pressure is constant.
B)ΔH is less than ΔU because of the pressure-volume work done by the gaseous products.
C)ΔH is less than ΔU because the atmosphere does pressure-volume work on the gaseous products.
D)ΔH equals ΔU because both are state functions.
E)ΔH is greater than ΔU because of the pressure-volume work done by the gaseous products.
2H2O(l)→ 2H2(g)+ O2(g)
A)ΔH is greater than ΔU because the pressure is constant.
B)ΔH is less than ΔU because of the pressure-volume work done by the gaseous products.
C)ΔH is less than ΔU because the atmosphere does pressure-volume work on the gaseous products.
D)ΔH equals ΔU because both are state functions.
E)ΔH is greater than ΔU because of the pressure-volume work done by the gaseous products.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
9
At constant pressure,the sign of q for the process CO2(s)→ CO2(g)is expected to be
A)positive,and the process is exothermic.
B)negative,and the process is exothermic.
C)impossible to predict.
D)positive,and the process is endothermic.
E)negative,and the process is endothermic.
A)positive,and the process is exothermic.
B)negative,and the process is exothermic.
C)impossible to predict.
D)positive,and the process is endothermic.
E)negative,and the process is endothermic.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
10
What is the kinetic energy of a 2000-lb car traveling at 59 miles per hour? (1 lb = 0.4536 kg,1 mi = 1.609 km)
A)3.2 × 10-7 J
B)1.5 × 106 J
C)5.3 × 1019 J
D)3.2 × 105 J
E)4.7 × 10-8 J
A)3.2 × 10-7 J
B)1.5 × 106 J
C)5.3 × 1019 J
D)3.2 × 105 J
E)4.7 × 10-8 J

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
11
The skydiver plunging to earth possesses what type(s)of energy?
A)potential energy only
B)internal energy only
C)kinetic energy only
D)kinetic energy,potential energy,and internal energy
E)kinetic energy and potential energy only
A)potential energy only
B)internal energy only
C)kinetic energy only
D)kinetic energy,potential energy,and internal energy
E)kinetic energy and potential energy only

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
12
The internal energy of a substance is defined as
A)the potential energy of all particles which make up the substance.
B)the kinetic energy of all particles which make up the substance.
C)the sum of the potential and kinetic energy of all particles which make up the substance.
D)the thermal energy of all particles which make up the substance.
E)the chemical energy of all particles which make up the substance.
A)the potential energy of all particles which make up the substance.
B)the kinetic energy of all particles which make up the substance.
C)the sum of the potential and kinetic energy of all particles which make up the substance.
D)the thermal energy of all particles which make up the substance.
E)the chemical energy of all particles which make up the substance.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
13
The energy associated with an object held above the surface of the earth is called _____.
A)heat
B)internal energy
C)temperature
D)kinetic energy
E)potential energy
A)heat
B)internal energy
C)temperature
D)kinetic energy
E)potential energy

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
14
The phrase "the heat absorbed or released by a system undergoing a physical or chemical change at constant pressure" is
A)the change in enthalpy of the system.
B)the change in internal energy of the system.
C)the definition of a state function.
D)the temperature change of the system.
E)a statement of Hess's law.
A)the change in enthalpy of the system.
B)the change in internal energy of the system.
C)the definition of a state function.
D)the temperature change of the system.
E)a statement of Hess's law.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following does not result in a change in the internal energy of the system?
A)work is done on the system
B)work is done on the surroundings
C)heat flows into the system
D)heat flows to the surroundings
E)none of the above
A)work is done on the system
B)work is done on the surroundings
C)heat flows into the system
D)heat flows to the surroundings
E)none of the above

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
16
Calculate ΔU of a gas for a process in which the gas absorbs 29 J of heat and does 31 J of work by expanding.
A)2 J
B)60 J
C)-60 J
D)0,because ΔU is a state function
E)-2 J
A)2 J
B)60 J
C)-60 J
D)0,because ΔU is a state function
E)-2 J

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
17
At constant pressure,the sign of q for the process H2O(l)→ H2O(s)is expected to be
A)positive,and the process is exothermic.
B)negative,and the process is endothermic.
C)impossible to predict.
D)negative,and the process is exothermic.
E)positive,and the process is endothermic.
A)positive,and the process is exothermic.
B)negative,and the process is endothermic.
C)impossible to predict.
D)negative,and the process is exothermic.
E)positive,and the process is endothermic.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
18
The energy associated with the motion of molecules in a gas is called _____.
A)heat
B)internal energy
C)temperature
D)kinetic energy
E)potential energy
A)heat
B)internal energy
C)temperature
D)kinetic energy
E)potential energy

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements is not true for an exothermic reaction?
A)The products have a higher heat content than the reactants.
B)The temperature of the reaction system increases.
C)The temperature of the surroundings increases.
D)Heat passes from the reaction system to the surroundings.
E)The enthalpy change for the reaction is negative.
A)The products have a higher heat content than the reactants.
B)The temperature of the reaction system increases.
C)The temperature of the surroundings increases.
D)Heat passes from the reaction system to the surroundings.
E)The enthalpy change for the reaction is negative.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
20
If q = 60 kJ and w = 96 kJ for a certain process,that process
A)requires a catalyst.
B)is endothermic.
C)occurs slowly.
D)is exothermic.
E)cannot occur.
A)requires a catalyst.
B)is endothermic.
C)occurs slowly.
D)is exothermic.
E)cannot occur.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
21
What is the quantity of heat evolved at constant pressure when 30.5 g H2O(l)is formed from the combustion of H2(g)and O2(g)?
H2(g)+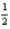
O2(g)→ H2O(l); ΔH° = -285.8 kJ
A)5.92 × 10-3 kJ
B)285.8 kJ
C)8.72 × 103 kJ
D)168 kJ
E)4.84 × 102 kJ
H2(g)+
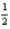
O2(g)→ H2O(l); ΔH° = -285.8 kJ
A)5.92 × 10-3 kJ
B)285.8 kJ
C)8.72 × 103 kJ
D)168 kJ
E)4.84 × 102 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
22
What is the change in enthalpy when 9.00 mol of sulfur trioxide decomposes to sulfur dioxide and oxygen gas?
2SO2(g)+ O2(g)→ 2SO3(g); ΔH° = 198 kJ
A)891 kJ
B)-198 kJ
C)-891 kJ
D)198 kJ
E)1782 kJ
2SO2(g)+ O2(g)→ 2SO3(g); ΔH° = 198 kJ
A)891 kJ
B)-198 kJ
C)-891 kJ
D)198 kJ
E)1782 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
23
When 56.8 g of lead reacts with 3.50 L of oxygen gas,measured at 1.00 atm and 25.0°C,60.1 kJ of heat is released at constant pressure.What is ΔH° for this reaction? (R = 0.0821 L • atm/(K • mol))
2Pb(s)+ O2(g)→ 2PbO(s)
A)-4.39 × 102 kJ
B)-8.7 × 101 kJ
C)-6.01 × 101 kJ
D)-2.19 × 102 kJ
E)-4.2 × 102 kJ
2Pb(s)+ O2(g)→ 2PbO(s)
A)-4.39 × 102 kJ
B)-8.7 × 101 kJ
C)-6.01 × 101 kJ
D)-2.19 × 102 kJ
E)-4.2 × 102 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
24
Under conditions of constant pressure,for which of the following reactions is the magnitude of pressure-volume work going to be smallest?
A)BaO(s)+ SO3(g)→ BaSO4(s)
B)2NO(g)+ O2(g)→ 2NO2(g)
C)2H2O2(l)→ 2H2O(l)+ O2(g)
D)2KClO3(s)→ 2KCl(s)+ 3O2(g)
E)H2(g)+ Cl2(g)→ 2HCl(g)
A)BaO(s)+ SO3(g)→ BaSO4(s)
B)2NO(g)+ O2(g)→ 2NO2(g)
C)2H2O2(l)→ 2H2O(l)+ O2(g)
D)2KClO3(s)→ 2KCl(s)+ 3O2(g)
E)H2(g)+ Cl2(g)→ 2HCl(g)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
25
Under conditions of constant pressure,for which of the following reactions is the magnitude of pressure-volume work going to be greatest?
A)BaO(s)+ SO3(g)→ BaSO4(s)
B)2NO(g)+ O2(g)→ 2NO2(g)
C)2H2O2(l)→ 2H2O(l)+ O2(g)
D)2KClO3(s)→ 2KCl(s)+ 3O2(g)
E)H2(g)+ Cl2(g)→ 2HCl(g)
A)BaO(s)+ SO3(g)→ BaSO4(s)
B)2NO(g)+ O2(g)→ 2NO2(g)
C)2H2O2(l)→ 2H2O(l)+ O2(g)
D)2KClO3(s)→ 2KCl(s)+ 3O2(g)
E)H2(g)+ Cl2(g)→ 2HCl(g)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
26
How much heat is evolved upon the complete oxidation of 6 g of aluminum at 25°C and 1 atm pressure? ( 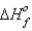 for Al2O3 is -1676 kJ/mol.)
for Al2O3 is -1676 kJ/mol.)
4Al(s)+ 3O2(g)→ 2Al2O3(s)
A)9.238 × 103 kJ
B)342.3 kJ
C)684.7 kJ
D)171.1 kJ
E)85.59 kJ
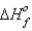 for Al2O3 is -1676 kJ/mol.)
for Al2O3 is -1676 kJ/mol.)4Al(s)+ 3O2(g)→ 2Al2O3(s)
A)9.238 × 103 kJ
B)342.3 kJ
C)684.7 kJ
D)171.1 kJ
E)85.59 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements is incorrect concerning the thermochemical equation below?
2SO3(g)→ 2SO2(g)+ O2(g); ΔH° = 198 kJ
A)The enthalpy of the reactants exceeds that of the products.
B)The reaction is endothermic.
C)For the reaction 2SO2(g)+ O2(g)→ 2SO3(g),ΔH° = -198 kJ.
D)The external pressure is 1 atm.
E)For every mole of SO3(g)consumed,99 kJ of heat at constant pressure is consumed as well.
2SO3(g)→ 2SO2(g)+ O2(g); ΔH° = 198 kJ
A)The enthalpy of the reactants exceeds that of the products.
B)The reaction is endothermic.
C)For the reaction 2SO2(g)+ O2(g)→ 2SO3(g),ΔH° = -198 kJ.
D)The external pressure is 1 atm.
E)For every mole of SO3(g)consumed,99 kJ of heat at constant pressure is consumed as well.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
28
How much heat is liberated at constant pressure if 0.833 g of calcium carbonate reacts with 59.7 mL of 0.251 M hydrochloric acid?
CaCO3(s)+ 2HCl(aq)→ CaCl2(aq)+ H2O(l)+ CO2(g); ΔH° = -15.2 kJ
A)0.113 kJ
B)0.24 kJ
C)12.6 kJ
D)0.126 kJ
E)3.81 kJ
CaCO3(s)+ 2HCl(aq)→ CaCl2(aq)+ H2O(l)+ CO2(g); ΔH° = -15.2 kJ
A)0.113 kJ
B)0.24 kJ
C)12.6 kJ
D)0.126 kJ
E)3.81 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
29
According to the following thermochemical equation,if 403.3 g of NO2 is produced,how much heat is released at constant pressure?
2NO(g)+ O2(g)→ 2NO2(g); ΔH° = -114.4 kJ
A)114.4 kJ
B)5.014 × 102 kJ
C)1.003 × 103 kJ
D)13.04 kJ
E)4.614 × 104 kJ
2NO(g)+ O2(g)→ 2NO2(g); ΔH° = -114.4 kJ
A)114.4 kJ
B)5.014 × 102 kJ
C)1.003 × 103 kJ
D)13.04 kJ
E)4.614 × 104 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
30
What quantity,in moles,of oxygen is consumed when 717.4 kJ of energy is evolved from the combustion of a mixture of H2(g)and O2(g)?
H2(g)+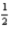
O2(g)→ H2O(l); ΔH° = -285.8 kJ
A)1.255 mol
B)2.519482 mol
C)0.1991 mol
D)1.755 mol
E)0.7559321 mol
H2(g)+
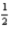
O2(g)→ H2O(l); ΔH° = -285.8 kJ
A)1.255 mol
B)2.519482 mol
C)0.1991 mol
D)1.755 mol
E)0.7559321 mol

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
31
Consider the following thermochemical equation:
N2(g)+ 2O2(g)→ 2NO2(g); ΔH° = 66.2 kJ
From this equation,we may conclude that 66.2 kJ is the quantity of heat that is
A)gained from the surroundings when 1 mol of NO2 is formed at constant pressure.
B)lost to the surroundings when 1 mol of NO2 is formed at constant pressure.
C)gained from the surroundings when 2 mol of NO2 is formed at constant pressure.
D)lost to the surroundings when 2 mol of NO2 is formed at constant pressure.
E)lost to the surroundings when 1 mol of O2 is consumed at constant pressure.
N2(g)+ 2O2(g)→ 2NO2(g); ΔH° = 66.2 kJ
From this equation,we may conclude that 66.2 kJ is the quantity of heat that is
A)gained from the surroundings when 1 mol of NO2 is formed at constant pressure.
B)lost to the surroundings when 1 mol of NO2 is formed at constant pressure.
C)gained from the surroundings when 2 mol of NO2 is formed at constant pressure.
D)lost to the surroundings when 2 mol of NO2 is formed at constant pressure.
E)lost to the surroundings when 1 mol of O2 is consumed at constant pressure.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
32
Given:
4AlCl3(s)+ 3O2(g)→ 2Al2O3(s)+ 6Cl2(g); ΔH = -529.0 kJ
Determine ΔH for the following thermochemical equation.
Cl2(g)+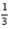 Al2O3(s)→
Al2O3(s)→ 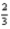 AlCl3(s)+
AlCl3(s)+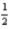 O2(g)
O2(g)
A)+264.5 kJ
B)+529.0 kJ
C)+88.2 kJ
D)+176.3 kJ
E)-176.3 kJ
4AlCl3(s)+ 3O2(g)→ 2Al2O3(s)+ 6Cl2(g); ΔH = -529.0 kJ
Determine ΔH for the following thermochemical equation.
Cl2(g)+
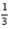 Al2O3(s)→
Al2O3(s)→ 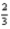 AlCl3(s)+
AlCl3(s)+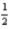 O2(g)
O2(g)A)+264.5 kJ
B)+529.0 kJ
C)+88.2 kJ
D)+176.3 kJ
E)-176.3 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
33
What is the change in enthalpy at 25°C and 1 atm for the production of 3.00 mol SnO(s)?
Sn(s)+ SnO2(s)→ 2SnO(s); ΔH° = 16.2 kJ
A)24.2 kJ
B)-16.2 kJ
C)16.2 kJ
D)5.4 kJ
E)24.3 kJ
Sn(s)+ SnO2(s)→ 2SnO(s); ΔH° = 16.2 kJ
A)24.2 kJ
B)-16.2 kJ
C)16.2 kJ
D)5.4 kJ
E)24.3 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
34
In a certain experiment,0.7000 mol of hydrogen gas reacted with 0.7000 mol of solid iodine at a constant 1 atm pressure,producing 1.4000 mol of solid hydrogen iodide and absorbing 36.9 kJ of heat in the process.Which of the following thermochemical equations correctly describes this experiment?
A)H2(g)+ I2(s)→ 2HI(s); ΔH° = -52.72 kJ
B)H2(g)+ I2(s)→ 2HI(s); ΔH° = 36.9 kJ
C)H2(g)+ I2(s)→ 2HI(s); ΔH° = -36.9 kJ
D)H2(g)+ I2(s)→ 2HI(s); ΔH° = 73.8 kJ
E)H2(g)+ I2(s)→ 2HI(s); ΔH° = 52.72 kJ
A)H2(g)+ I2(s)→ 2HI(s); ΔH° = -52.72 kJ
B)H2(g)+ I2(s)→ 2HI(s); ΔH° = 36.9 kJ
C)H2(g)+ I2(s)→ 2HI(s); ΔH° = -36.9 kJ
D)H2(g)+ I2(s)→ 2HI(s); ΔH° = 73.8 kJ
E)H2(g)+ I2(s)→ 2HI(s); ΔH° = 52.72 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statements is false concerning the reaction of hydrogen gas and oxygen gas given below?
H2(g)+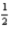
O2(g)→ H2O(l); ΔH = -285.8 kJ
A)Per mole of O2,the change in enthalpy is -571.6 kJ.
B)The value -571.6 kJ pertains to 1 mol of liquid water.
C)If the equation is reversed,ΔH becomes +285.8 kJ.
D)If the equation is multiplied by 2,ΔH becomes -571.6 kJ.
E)For the reaction H2(g)+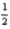 O2(g)→ H2O(g),ΔH is not equal to -285.8 kJ.
O2(g)→ H2O(g),ΔH is not equal to -285.8 kJ.
H2(g)+
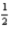
O2(g)→ H2O(l); ΔH = -285.8 kJ
A)Per mole of O2,the change in enthalpy is -571.6 kJ.
B)The value -571.6 kJ pertains to 1 mol of liquid water.
C)If the equation is reversed,ΔH becomes +285.8 kJ.
D)If the equation is multiplied by 2,ΔH becomes -571.6 kJ.
E)For the reaction H2(g)+
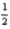 O2(g)→ H2O(g),ΔH is not equal to -285.8 kJ.
O2(g)→ H2O(g),ΔH is not equal to -285.8 kJ.
Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
36
Given: 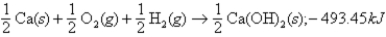 what is ΔH for the following thermochemical equation?
what is ΔH for the following thermochemical equation? 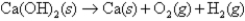
A)986.9 kJ
B)-986.9 kJ
C)-139 MJ
D)-2320 kJ
E)-38.7 kJ
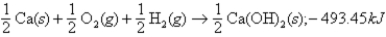 what is ΔH for the following thermochemical equation?
what is ΔH for the following thermochemical equation? 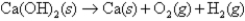
A)986.9 kJ
B)-986.9 kJ
C)-139 MJ
D)-2320 kJ
E)-38.7 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following sentences accurately describes the thermochemical equation given below?
2Ag(s)+ F2(g)→ 2AgF(s); ΔH = -409.2 kJ
A)If 2 mol of silver metal react with 1 mol of fluorine gas at constant volume,2 mol of solid sodium fluoride is produced and 409.2 kJ of heat is consumed.
B)If 2 atoms of silver metal react with 1 molecule of fluorine gas at constant pressure,2 formula units of solid sodium fluoride are produced and 409.2 kJ of heat is released.
C)If 2 atoms of silver metal react with 1 molecule of fluorine gas at constant pressure,2 formula units of solid sodium fluoride are produced and 409.2 kJ of heat is consumed.
D)If 2 mol of silver metal react with 1 mol of fluorine gas at constant pressure,2 mol of solid sodium fluoride is produced and 409.2 kJ of heat is consumed.
E)If 2 mol of silver metal react with 1 mol of fluorine gas at constant pressure,2 mol of solid sodium fluoride is produced and 409.2 kJ of heat is released.
2Ag(s)+ F2(g)→ 2AgF(s); ΔH = -409.2 kJ
A)If 2 mol of silver metal react with 1 mol of fluorine gas at constant volume,2 mol of solid sodium fluoride is produced and 409.2 kJ of heat is consumed.
B)If 2 atoms of silver metal react with 1 molecule of fluorine gas at constant pressure,2 formula units of solid sodium fluoride are produced and 409.2 kJ of heat is released.
C)If 2 atoms of silver metal react with 1 molecule of fluorine gas at constant pressure,2 formula units of solid sodium fluoride are produced and 409.2 kJ of heat is consumed.
D)If 2 mol of silver metal react with 1 mol of fluorine gas at constant pressure,2 mol of solid sodium fluoride is produced and 409.2 kJ of heat is consumed.
E)If 2 mol of silver metal react with 1 mol of fluorine gas at constant pressure,2 mol of solid sodium fluoride is produced and 409.2 kJ of heat is released.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
38
What is the change in enthalpy at 25°C and 1 atm for the reaction of 5.00 mol of elemental iron with excess oxygen gas?
4Fe(s)+ 3O2(g)→ 2Fe2O3(s); ΔH° = -1651 kJ
A)−1651 kJ
B)2751 kJ
C)206 kJ
D)2063 kJ
E)−412.8 kJ
4Fe(s)+ 3O2(g)→ 2Fe2O3(s); ΔH° = -1651 kJ
A)−1651 kJ
B)2751 kJ
C)206 kJ
D)2063 kJ
E)−412.8 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
39
Given the thermochemical equation
2Al(s)+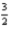
O2(g)→ Al2O3(s); ΔH = -1676 kJ
Find ΔH for the following reaction.
2Al2O3(s)→ 4Al(s)+ 3O2(g)
A)838 kJ
B)1676 kJ
C)-1676 kJ
D)3352 kJ
E)-838 kJ
2Al(s)+
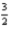
O2(g)→ Al2O3(s); ΔH = -1676 kJ
Find ΔH for the following reaction.
2Al2O3(s)→ 4Al(s)+ 3O2(g)
A)838 kJ
B)1676 kJ
C)-1676 kJ
D)3352 kJ
E)-838 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
40
What mass of oxygen is consumed when 285.5 kJ of energy is evolved from the combustion of a mixture of H2(g)and O2(g)?
H2(g)+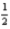
O2(g)→ H2O(l); ΔH° = -285.8 kJ
A)15.98 g
B)31.96 g
C)0.01564 g
D)31.98 g
E)0.01679 g
H2(g)+
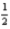
O2(g)→ H2O(l); ΔH° = -285.8 kJ
A)15.98 g
B)31.96 g
C)0.01564 g
D)31.98 g
E)0.01679 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
41
A 3.540-g sample of an unknown metal M is burned in the presence of excess oxygen,producing the oxide M2O3(s)and liberating 18.56 kJ of heat at constant pressure.What is the identity of the metal?
4M(s)+ 3O2(g)→ 2M2O3(s)
Substance
ΔH°f (kJ/mol)
Yb2O3(s)
-1814.6
Tb2O3(s)
-1865.2
Sm2O3(s)
-1823.0
Sc2O3(s)
-1908.8
Y2O3(s)
-1905.3
A)Sm
B)Yb
C)Y
D)Sc
E)Tb
4M(s)+ 3O2(g)→ 2M2O3(s)
Substance
ΔH°f (kJ/mol)
Yb2O3(s)
-1814.6
Tb2O3(s)
-1865.2
Sm2O3(s)
-1823.0
Sc2O3(s)
-1908.8
Y2O3(s)
-1905.3
A)Sm
B)Yb
C)Y
D)Sc
E)Tb

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
42
A 1.67-g sample of solid silver reacted in excess chlorine gas to give a 2.21-g sample of pure solid AgCl.The heat given off in this reaction was 1.96 kJ at constant pressure.Given this information,what is the enthalpy of formation of AgCl(s)?
A)127 kJ/mol
B)-63.5 kJ/mol
C)12.7 kJ/mol
D)1.96 kJ/mol
E)196 kJ/mol
A)127 kJ/mol
B)-63.5 kJ/mol
C)12.7 kJ/mol
D)1.96 kJ/mol
E)196 kJ/mol

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
43
At constant pressure and 25°C,what is ΔH° for the following reaction
2C2H6(g)+ 7O2(g)→ 4CO2(g)+ H2O(l)
If the complete consumption of 38.2 g of C2H6 liberates 1981 kJ of heat energy?
A)3120 kJ
B)1560 kJ
C)5030 kJ
D)2510 kJ
E)784.05 kJ
2C2H6(g)+ 7O2(g)→ 4CO2(g)+ H2O(l)
If the complete consumption of 38.2 g of C2H6 liberates 1981 kJ of heat energy?
A)3120 kJ
B)1560 kJ
C)5030 kJ
D)2510 kJ
E)784.05 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
44
The reaction of iron with hydrochloric acid is represented by the following thermochemical equation.
Fe(s)+ 2HCl(aq)→ FeCl2(aq)+ H2(g); ΔH° = -87.9 kJ
How much heat is liberated at constant pressure if 0.154 g of iron reacts with 25.7 mL of 0.358 M HCl?
A)1.85 kJ
B)13.5 kJ
C)0.242 kJ
D)0.404 kJ
E)87.9 kJ
Fe(s)+ 2HCl(aq)→ FeCl2(aq)+ H2(g); ΔH° = -87.9 kJ
How much heat is liberated at constant pressure if 0.154 g of iron reacts with 25.7 mL of 0.358 M HCl?
A)1.85 kJ
B)13.5 kJ
C)0.242 kJ
D)0.404 kJ
E)87.9 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
45
A 8.22-g sample of solid calcium reacted in excess fluorine gas to give a 16-g sample of pure solid CaF2.The heat given off in this reaction was 251 kJ at constant pressure.Given this information,what is the enthalpy of formation of CaF2(s)?
A)251 kJ/mol
B)250 kJ/mol
C)-1.23 × 103 kJ/mol
D)-613 kJ/mol
E)1.23 × 103 kJ/mol
A)251 kJ/mol
B)250 kJ/mol
C)-1.23 × 103 kJ/mol
D)-613 kJ/mol
E)1.23 × 103 kJ/mol

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
46
How much heat is released at constant pressure if 12.3 mL of 0.161 M silver nitrate is mixed with 41.7 mL of 0.493 M potassium chloride?
AgNO3(aq)+ KCl(aq)→ AgCl(s)+ KNO3(aq); ΔH° = -65.5 kJ
A)0.129 kJ
B)32.2 kJ
C)1.34 kJ
D)10.5 kJ
E)1.47 kJ
AgNO3(aq)+ KCl(aq)→ AgCl(s)+ KNO3(aq); ΔH° = -65.5 kJ
A)0.129 kJ
B)32.2 kJ
C)1.34 kJ
D)10.5 kJ
E)1.47 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
47
When 22.0 mL of liquid benzene (C6H6,d = 0.879 g/mL)reacts with 34.2 L of oxygen gas,measured at 1.00 atm pressure and 25°C,6.09 × 102 kJ of heat is released at constant pressure.What is ΔH° for the following reaction? (R = 0.0821 L • atm/(K • mol))
2C6H6(l)+ 15O2(g)→ 12CO2(g)+ 6H2O(l)
A)-2.84 × 101 kJ
B)-3.7 × 102 kJ
C)-4.92 × 103 kJ
D)-4.36 × 102 kJ
E)-6.53 × 103 kJ
2C6H6(l)+ 15O2(g)→ 12CO2(g)+ 6H2O(l)
A)-2.84 × 101 kJ
B)-3.7 × 102 kJ
C)-4.92 × 103 kJ
D)-4.36 × 102 kJ
E)-6.53 × 103 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
48
The specific heat capacity of water(liquid)is 4.18 J/g⋅°C.What is the molar specific heat capacity of this substance? The molar mass of water is 18.01 g/mol.
A)75.2 J/mol⋅°C
B)0.232 J/mol⋅°C
C)4.303 J/mol⋅°C
D)4.18 J/mol⋅°C
E)0.239 J/mol⋅°C
A)75.2 J/mol⋅°C
B)0.232 J/mol⋅°C
C)4.303 J/mol⋅°C
D)4.18 J/mol⋅°C
E)0.239 J/mol⋅°C

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
49
The quantity of heat required to raise the temperature of a sample of a substance by 1°C is the sample's
A)work.
B)calorimetry.
C)heat capacity.
D)specific heat.
E)enthalpy.
A)work.
B)calorimetry.
C)heat capacity.
D)specific heat.
E)enthalpy.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
50
How much heat is liberated at constant pressure when 97.7 g of calcium oxide reacts with 29.0 L of carbon dioxide gas,measured at 1.00 atm pressure and 25.0°C? (R = 0.0821 L • atm/(K • mol))
CaO(s)+ CO2(g)→ CaCO3(s); ΔH° = -178.3 kJ
A)-3.11 × 102 kJ
B)-1.74 × 104 kJ
C)-5.22 × 102 kJ
D)-2.11 × 102 kJ
E)-5.17 × 103 kJ
CaO(s)+ CO2(g)→ CaCO3(s); ΔH° = -178.3 kJ
A)-3.11 × 102 kJ
B)-1.74 × 104 kJ
C)-5.22 × 102 kJ
D)-2.11 × 102 kJ
E)-5.17 × 103 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
51
It is relatively easy to change the temperature of a substance that
A)is very massive.
B)is an insulator.
C)has a high specific heat capacity.
D)has a low specific heat capacity.
E)is brittle.
A)is very massive.
B)is an insulator.
C)has a high specific heat capacity.
D)has a low specific heat capacity.
E)is brittle.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
52
The reaction of iron with hydrochloric acid is represented by the following thermochemical equation.
Fe(s)+ 2HCl(aq)→ FeCl2(aq)+ H2(g); ΔH° = -87.9 kJ
In which of the following experiments would the temperature rise the most?
A)2.2 g of Fe added to 1.0 L of 0.03 M HCl
B)1.1 g of Fe added to 1.0 L of 0.02 M HCl
C)4.5 g of Fe added to 1.0 L of 0.03 M HCl
D)1.1 g of Fe added to 1.0 L of 0.04 M HCl
E)0.56 g of Fe added to 1.0 L of 0.02 M HCl
Fe(s)+ 2HCl(aq)→ FeCl2(aq)+ H2(g); ΔH° = -87.9 kJ
In which of the following experiments would the temperature rise the most?
A)2.2 g of Fe added to 1.0 L of 0.03 M HCl
B)1.1 g of Fe added to 1.0 L of 0.02 M HCl
C)4.5 g of Fe added to 1.0 L of 0.03 M HCl
D)1.1 g of Fe added to 1.0 L of 0.04 M HCl
E)0.56 g of Fe added to 1.0 L of 0.02 M HCl

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
53
The reaction of iron with hydrochloric acid is represented by the following thermochemical equation.
Fe(s)+ 2HCl(aq)→ FeCl2(aq)+ H2(g); ΔH° = -87.9 kJ
If,in a particular experiment,1.56 kJ of heat was released at constant pressure,what volume of H2(g),measured at STP,was produced? (R = 0.0821 L • atm/(K • mol))
A)1.38 × 103 L
B)0.434 L
C)22.4 L
D)1.26 × 103 L
E)0.397 L
Fe(s)+ 2HCl(aq)→ FeCl2(aq)+ H2(g); ΔH° = -87.9 kJ
If,in a particular experiment,1.56 kJ of heat was released at constant pressure,what volume of H2(g),measured at STP,was produced? (R = 0.0821 L • atm/(K • mol))
A)1.38 × 103 L
B)0.434 L
C)22.4 L
D)1.26 × 103 L
E)0.397 L

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
54
The heat required to raise the temperature of 54.94 g of manganese by 1°C is called its
A)heat of vaporization.
B)specific heat.
C)heat of fusion.
D)entropy.
E)molar heat capacity.
A)heat of vaporization.
B)specific heat.
C)heat of fusion.
D)entropy.
E)molar heat capacity.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
55
The units for specific heat are
A)J/(g ∙ °C).
B)(J ∙ °C).
C)J/g.
D)J/°C.
E)(J ∙ g).
A)J/(g ∙ °C).
B)(J ∙ °C).
C)J/g.
D)J/°C.
E)(J ∙ g).

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
56
The units for heat capacity are
A)J/g.
B)(J ∙ g).
C)J/°C.
D)(J ∙ °C).
E)J/(g ∙ °C).
A)J/g.
B)(J ∙ g).
C)J/°C.
D)(J ∙ °C).
E)J/(g ∙ °C).

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
57
When 13.8 mL of 0.870 M lead(II)nitrate reacts with 90.0 mL of 0.777 M sodium chloride,0.279 kJ of heat is released at constant pressure.What is ΔH° for this reaction?
Pb(NO3)2(aq)+ 2NaCl(aq)→ PbCl2(s)+ 2NaNO3(aq)
A)23.3 kJ
B)4 kJ
C)1.84 kJ
D)3.41 kJ
E)8 kJ
Pb(NO3)2(aq)+ 2NaCl(aq)→ PbCl2(s)+ 2NaNO3(aq)
A)23.3 kJ
B)4 kJ
C)1.84 kJ
D)3.41 kJ
E)8 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
58
A 100 g sample of each of the following metals is heated from 35°C to 45°C.Which metal absorbs the lowest amount of heat energy?
Metal
Specific Heat
Copper
0)385 J/(g ∙ °C)
Magnesium
1)02 J/(g ∙ °C)
Mercury
0)138 J/(g ∙ °C)
Silver
0)237 J/(g ∙ °C)
Lead
0)129 J/(g ∙ °C)
A)lead
B)magnesium
C)silver
D)mercury
E)copper
Metal
Specific Heat
Copper
0)385 J/(g ∙ °C)
Magnesium
1)02 J/(g ∙ °C)
Mercury
0)138 J/(g ∙ °C)
Silver
0)237 J/(g ∙ °C)
Lead
0)129 J/(g ∙ °C)
A)lead
B)magnesium
C)silver
D)mercury
E)copper

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
59
Two metals of equal mass with different heat capacities are subjected to the same amount of heat.Which undergoes the smaller change in temperature?
A)The metal with the higher heat capacity undergoes the smaller change in temperature.
B)Both undergo the same change in temperature.
C)You need to know the initial temperatures of the metals.
D)You need to know which metals you have.
E)The metal with the lower heat capacity undergoes the smaller change in temperature.
A)The metal with the higher heat capacity undergoes the smaller change in temperature.
B)Both undergo the same change in temperature.
C)You need to know the initial temperatures of the metals.
D)You need to know which metals you have.
E)The metal with the lower heat capacity undergoes the smaller change in temperature.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
60
The molar heat capacity of gaseous heptane at 25.0°C is 165.2 J/(mol ∙ °C).What is its specific heat?
A)0.6065 J/(g ∙ °C)
B)1.648 J/(g ∙ °C)
C)6.041 × 10-5 J/(g ∙ °C)
D)165.2 J/(g ∙ °C)
E)1.655 × 104 J/(g ∙ °C)
A)0.6065 J/(g ∙ °C)
B)1.648 J/(g ∙ °C)
C)6.041 × 10-5 J/(g ∙ °C)
D)165.2 J/(g ∙ °C)
E)1.655 × 104 J/(g ∙ °C)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following processes will result in the lowest final temperature of the metal-water mixture at equilibrium? The specific heat of cobalt is 0.421 J/(g ∙ °C).
A)the addition of 100 g of cobalt at 95°C to 80 mL of water at 25°C in an insulated container
B)the addition of 100 g of cobalt at 95°C to 100 mL of water at 25°C in an insulated container
C)the addition of 100 g of cobalt at 95°C to 40 mL of water at 25°C in an insulated container
D)the addition of 100 g of cobalt at 95°C to 20 mL of water at 25°C in an insulated container
E)the addition of 100 g of cobalt at 95°C to 60 mL of water at 25°C in an insulated container
A)the addition of 100 g of cobalt at 95°C to 80 mL of water at 25°C in an insulated container
B)the addition of 100 g of cobalt at 95°C to 100 mL of water at 25°C in an insulated container
C)the addition of 100 g of cobalt at 95°C to 40 mL of water at 25°C in an insulated container
D)the addition of 100 g of cobalt at 95°C to 20 mL of water at 25°C in an insulated container
E)the addition of 100 g of cobalt at 95°C to 60 mL of water at 25°C in an insulated container

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
62
Exactly 209.2 J will raise the temperature of 10.0 g of a metal from 25.0°C to 60.0°C.What is the specific heat capacity of the metal?
A)0.598 J/(g ∙ °C)
B)1.67 J/(g ∙ °C)
C)14.7 J/(g ∙ °C)
D)50.0 J/(g ∙ °C)
E)none of these
A)0.598 J/(g ∙ °C)
B)1.67 J/(g ∙ °C)
C)14.7 J/(g ∙ °C)
D)50.0 J/(g ∙ °C)
E)none of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
63
A 110.0-g sample of metal at 82.00°C is added to 110.0 g of H2O(l)at 27.00°C in an insulated container.The temperature rises to 30.56°C.Neglecting the heat capacity of the container,what is the specific heat of the metal? The specific heat of H2O(l)is 4.18 J/(g ∙ °C).
A)4.18 J/(g ∙ °C)
B)60.4 J/(g ∙ °C)
C)0.289 J/(g ∙ °C)
D)0.28 J/(g ∙ °C)
E)14.4 J/(g ∙ °C)
A)4.18 J/(g ∙ °C)
B)60.4 J/(g ∙ °C)
C)0.289 J/(g ∙ °C)
D)0.28 J/(g ∙ °C)
E)14.4 J/(g ∙ °C)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
64
A 58.7-g sample of nickel (s = 0.443 J/(g ∙ °C)),initially at 147.4°C,is placed in an insulated vessel containing 184.0 g of water (s = 4.18 J/(g ∙ °C)),initially at 29.9°C.Once equilibrium is reached,what is the final temperature of the metal-water mixture? Neglect the heat capacity of the vessel.
A)33.7°C
B)58.3°C
C)25.8°C
D)88.7°C
E)41.2°C
A)33.7°C
B)58.3°C
C)25.8°C
D)88.7°C
E)41.2°C

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
65
Given:
Fe2O3(s)+ 3CO(g)→ 2Fe(s)+ 3CO2(g); ΔH° = -26.8 kJ
FeO(s)+ CO(g)→ Fe(s)+ CO2(g); ΔH° = -16.5 kJ
Determine ΔH° for the following thermochemical equation.
Fe2O3(s)+ CO(g)→ 2FeO(s)+ CO2(g)
A)6.2 kJ
B)10.3 kJ
C)22.7 kJ
D)-10.3 kJ
E)-43.3 kJ
Fe2O3(s)+ 3CO(g)→ 2Fe(s)+ 3CO2(g); ΔH° = -26.8 kJ
FeO(s)+ CO(g)→ Fe(s)+ CO2(g); ΔH° = -16.5 kJ
Determine ΔH° for the following thermochemical equation.
Fe2O3(s)+ CO(g)→ 2FeO(s)+ CO2(g)
A)6.2 kJ
B)10.3 kJ
C)22.7 kJ
D)-10.3 kJ
E)-43.3 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
66
When 0.0500 mol of HCl(aq)is reacted with 0.0500 mol of NaOH(aq)in 50.0 mL of water,the temperature of the solution increases by 5.99°C.What is the enthalpy of reaction for the following thermochemical equation?
HCl(aq)+ NaOH(aq)→ NaCl(aq)+ H2O(l)
Assume that the heat capacity of the solution and calorimeter is 465.4 J/°C.
A)0.139 kJ
B)55.8 kJ
C)2.791 kJ
D)-55.8 kJ
E)2.79 kJ
HCl(aq)+ NaOH(aq)→ NaCl(aq)+ H2O(l)
Assume that the heat capacity of the solution and calorimeter is 465.4 J/°C.
A)0.139 kJ
B)55.8 kJ
C)2.791 kJ
D)-55.8 kJ
E)2.79 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
67
A 500-cm3 sample of 1.0 M NaOH(aq)is added to 500 cm3 of 1.0 M HCl(aq)in a Styrofoam cup,and the solution is quickly stirred.The rise in temperature (ΔT1)is measured.The experiment is repeated using 100 cm3 of each solution,and the rise in temperature (ΔT2)is measured.What conclusion can you draw about ΔT1 and ΔT2?
HCl(aq)+ NaOH(aq)→ H2O(l)+ NaCl(aq); ΔH° = -55.8 kJ
A)ΔT2 is greater than ΔT1.
B)ΔT2 is equal to ΔT1.
C)ΔT1 is five times as large as ΔT2.
D)ΔT1 is less than ΔT2.
E)ΔT2 is five times as large as ΔT1.
HCl(aq)+ NaOH(aq)→ H2O(l)+ NaCl(aq); ΔH° = -55.8 kJ
A)ΔT2 is greater than ΔT1.
B)ΔT2 is equal to ΔT1.
C)ΔT1 is five times as large as ΔT2.
D)ΔT1 is less than ΔT2.
E)ΔT2 is five times as large as ΔT1.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
68
A 84.8-g piece of silver (s = 0.237 J/(g ∙ °C)),initially at 268.0°C,is added to 128.4 g of a liquid,initially at 24.9°C,in an insulated container.The final temperature of the metal-liquid mixture at equilibrium is 39.6°C.What is the identity of the liquid? Neglect the heat capacity of the container.
A)methanol (s = 2.53 J/(g ∙ °C))
B)ethanol (s = 2.43 J/(g ∙ °C))
C)hexane (s = 2.27 J/(g ∙ °C))
D)water (s = 4.18 J/(g ∙ °C))
E)acetone (s = 2.15 J/(g ∙ °C))
A)methanol (s = 2.53 J/(g ∙ °C))
B)ethanol (s = 2.43 J/(g ∙ °C))
C)hexane (s = 2.27 J/(g ∙ °C))
D)water (s = 4.18 J/(g ∙ °C))
E)acetone (s = 2.15 J/(g ∙ °C))

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
69
How much heat must be applied to a 40.2-g sample of gold (s = 0.129 J/(g ∙ °C))in order to raise its temperature from 28.2°C to 351.5°C?
A)1.82 × 103 J
B)1.68 × 103 J
C)1.46 × 102 J
D)1.3 × 104 J
E)4.17 × 101 J
A)1.82 × 103 J
B)1.68 × 103 J
C)1.46 × 102 J
D)1.3 × 104 J
E)4.17 × 101 J

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
70
How much heat is gained by copper when 77.5 g of copper is warmed from 21.4°C to 75.1°C? The specific heat of copper is 0.385 J/(g ∙ °C).
A)6.39 × 102 J
B)28.91 J
C)20.67 J
D)1.6 × 103 J
E)2.24 × 103 J
A)6.39 × 102 J
B)28.91 J
C)20.67 J
D)1.6 × 103 J
E)2.24 × 103 J

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
71
When 9.42 g of methane (CH4)is burned in a bomb calorimeter (heat capacity = 2.677 × 103 J/°C),the temperature rises from 24.00 to 27.08°C.How much heat is absorbed by the calorimeter?
CH4(g)+ 2O2(g)→ CO2(g)+ 2H2O(l); ΔH° = -1283.8 kJ
A)745 kJ
B)4.84 × 103 kJ
C)8.24 kJ
D)753 kJ
E)1.28 × 103 kJ
CH4(g)+ 2O2(g)→ CO2(g)+ 2H2O(l); ΔH° = -1283.8 kJ
A)745 kJ
B)4.84 × 103 kJ
C)8.24 kJ
D)753 kJ
E)1.28 × 103 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
72
When 50.0 mL of 1.27 M of HCl(aq)is combined with 50.0 mL of 1.32 M of NaOH(aq)in a coffee-cup calorimeter,the temperature of the solution increases by 8°C.What is the change in enthalpy for this balanced reaction?
HCl(aq)+ NaOH(aq)→ NaCl(aq)+ H2O(l)
Assume that the solution density is 1.00 g/mL and the specific heat capacity of the solution is 4.18 J/g⋅°C.
A)-55.8 kJ
B)55.8 kJ
C)51.5 kJ
D)-51.5 kJ
E)-26.8 kJ
HCl(aq)+ NaOH(aq)→ NaCl(aq)+ H2O(l)
Assume that the solution density is 1.00 g/mL and the specific heat capacity of the solution is 4.18 J/g⋅°C.
A)-55.8 kJ
B)55.8 kJ
C)51.5 kJ
D)-51.5 kJ
E)-26.8 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
73
A 86.9-g sample of chromium (s = 0.447 J/(g ∙ °C)),initially at 338.33°C,is added to an insulated vessel containing 189.9 g of water (s = 4.18 J/(g ∙ °C)),initially at 16.17°C.At equilibrium,the final temperature of the metal-water mixture is 28.06°C.How much heat was absorbed by the water? The heat capacity of the vessel is 0.220 kJ/°C.
A)9.43 kJ
B)6.82 kJ
C)12 kJ
D)15.2 kJ
E)112 kJ
A)9.43 kJ
B)6.82 kJ
C)12 kJ
D)15.2 kJ
E)112 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
74
In a bomb calorimeter,reactions are carried out
A)at 1 atm pressure and 0°C.
B)at a constant pressure.
C)at a constant volume.
D)at a constant pressure and 25°C.
E)at 1 atm pressure and 25°C.
A)at 1 atm pressure and 0°C.
B)at a constant pressure.
C)at a constant volume.
D)at a constant pressure and 25°C.
E)at 1 atm pressure and 25°C.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
75
A bomb calorimeter has a heat capacity of 2.47 kJ/K.When a 0.106-g sample of a certain hydrocarbon was burned in this calorimeter,the temperature increased by 2.14 K.Calculate the energy of combustion for 1 g of the hydrocarbon.
A)-5.29 J/g
B)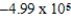 J/g
J/g
C)-0.120 J/g
D)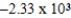 J/g
J/g
E)-0.560 J/g
A)-5.29 J/g
B)
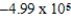 J/g
J/gC)-0.120 J/g
D)
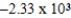 J/g
J/gE)-0.560 J/g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
76
Given:
Pb(s)+ PbO2(s)+ 2H2SO4(l)→ 2PbSO4(s)+ 2H2O(l); ΔH° = -509.2 kJ
SO3(g)+ H2O(l)→ H2SO4(l); ΔH° = -130.kJ
Determine ΔH° for the following thermochemical equation.
Pb(s)+ PbO2(s)+ 2SO3(g)→ 2PbSO4(s)
A)3.77 × 103 kJ
B)-521 kJ
C)-3.77 × 103 kJ
D)-639 kJ
E)-769 kJ
Pb(s)+ PbO2(s)+ 2H2SO4(l)→ 2PbSO4(s)+ 2H2O(l); ΔH° = -509.2 kJ
SO3(g)+ H2O(l)→ H2SO4(l); ΔH° = -130.kJ
Determine ΔH° for the following thermochemical equation.
Pb(s)+ PbO2(s)+ 2SO3(g)→ 2PbSO4(s)
A)3.77 × 103 kJ
B)-521 kJ
C)-3.77 × 103 kJ
D)-639 kJ
E)-769 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
77
Using the following data,calculate the standard enthalpy of reaction for the coal gasification process 2C(s)+ 2H2O(g)→ CH4(g)+ CO2(g).
C(s)+ H2O(g)→ CO(g)+ H2(g); ΔH° = +131.3 kJ
CO(g)+ H2O(g)→ CO2(g)+ H2(g); ΔH° = -41.2 kJ
CO(g)+ 3H2(g)→ CH4(g)+ H2O(g); ΔH° = -206.1 kJ
A)-116.0 kJ
B)378.6 kJ
C)+15.3 kJ
D)-157.2 kJ
E)-378.6 kJ
C(s)+ H2O(g)→ CO(g)+ H2(g); ΔH° = +131.3 kJ
CO(g)+ H2O(g)→ CO2(g)+ H2(g); ΔH° = -41.2 kJ
CO(g)+ 3H2(g)→ CH4(g)+ H2O(g); ΔH° = -206.1 kJ
A)-116.0 kJ
B)378.6 kJ
C)+15.3 kJ
D)-157.2 kJ
E)-378.6 kJ

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
78
The overall chemical equation resulting from the sum of the following three steps is
2C(s)+ 2H2O(g)→ 2CO(g)+ 2H2(g)
CO(g)+ H2O(g)→ CO2(g)+ H2(g)
CO(g)+ 3H2(g)→ CH4(g)+ H2O(g)
A)2C(s)+ 2H2O(g)→ CO2(g)+ CH4(g)
B)2C(s)+ 3H2O(g)→ CO(g)+ CO2(g)+ 3H2(g)
C)2C(s)+ H2O(g)+ H2(g)→ CO(g)+ CH4(g)
D)2CO(g)+ 2H2(g)→ CH4(g)+ CO2(g)
E)2C(s)+ CH4(g)+ 3H2O(g)→ 3CO(g)+ 5H2(g)
2C(s)+ 2H2O(g)→ 2CO(g)+ 2H2(g)
CO(g)+ H2O(g)→ CO2(g)+ H2(g)
CO(g)+ 3H2(g)→ CH4(g)+ H2O(g)
A)2C(s)+ 2H2O(g)→ CO2(g)+ CH4(g)
B)2C(s)+ 3H2O(g)→ CO(g)+ CO2(g)+ 3H2(g)
C)2C(s)+ H2O(g)+ H2(g)→ CO(g)+ CH4(g)
D)2CO(g)+ 2H2(g)→ CH4(g)+ CO2(g)
E)2C(s)+ CH4(g)+ 3H2O(g)→ 3CO(g)+ 5H2(g)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
79
Combustion of 3.65 g of liquid benzene (C6H6)causes a temperature rise of 21.1°C in a constant-pressure calorimeter that has a heat capacity of 7.23 kJ/°C.What is ΔH for the following reaction?
C6H6(l)+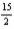 O2(g)→ 6CO2(g)+ 3H2O(l)
O2(g)→ 6CO2(g)+ 3H2O(l)
A)150 kJ/mol
B)41.8 kJ/mol
C)-41.8 kJ/mol
D)-3.27× 103 kJ/mol
E)152 kJ/mol
C6H6(l)+
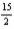 O2(g)→ 6CO2(g)+ 3H2O(l)
O2(g)→ 6CO2(g)+ 3H2O(l)A)150 kJ/mol
B)41.8 kJ/mol
C)-41.8 kJ/mol
D)-3.27× 103 kJ/mol
E)152 kJ/mol

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following processes will result in the lowest final temperature of the metal-water mixture at equilibrium?
A)the addition of 100 g of silver (s = 0.237 J/(g ∙ °C))at 95°C to 100 mL of water at 25°C in an insulated container
B)the addition of 100 g of cobalt (s = 0.418 J/(g ∙ °C))at 95°C to 100 mL of water at 25°C in an insulated container
C)the addition of 100 g of chromium (s = 0.447 J/(g ∙ °C))at 95°C to 100 mL of water at 25°C in an insulated container
D)the addition of 100 g of copper (s = 0.385 J/(g ∙ °C))at 95°C to 100 mL of water at 25°C in an insulated container
E)the addition of 100 g of gold (s = 0.129 J/(g ∙ °C))at 95°C to 100 mL of water at 25°C in an insulated container
A)the addition of 100 g of silver (s = 0.237 J/(g ∙ °C))at 95°C to 100 mL of water at 25°C in an insulated container
B)the addition of 100 g of cobalt (s = 0.418 J/(g ∙ °C))at 95°C to 100 mL of water at 25°C in an insulated container
C)the addition of 100 g of chromium (s = 0.447 J/(g ∙ °C))at 95°C to 100 mL of water at 25°C in an insulated container
D)the addition of 100 g of copper (s = 0.385 J/(g ∙ °C))at 95°C to 100 mL of water at 25°C in an insulated container
E)the addition of 100 g of gold (s = 0.129 J/(g ∙ °C))at 95°C to 100 mL of water at 25°C in an insulated container

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck


